CytoBead Assays – A state of the art combination of cell-based immunofluorescence and microparticle technology for simultaneous screening and differentiation in autoimmune diagnostics
Autoimmune diseases affect approximately 5 % of the population of developed countries with an increasing incidence. Analysis of disease-associated autoantibodies (AAb) plays a significant role in the differential diagnosis thereof. Indirect immunofluorescence (IIF) has been established as the gold standard for AAb screening in particular for systemic rheumatic diseases. In the recommended two-tier approach for antibody serology, confirmatory testing by molecular assay techniques such as ELISA is required to confirm positive findings by screening using IIF. To cope with the constantly increasing demand for AAb testing, new efficient diagnostic approaches are required. Thus, a new generation of IIF assays have been developed to combine screening and confirmatory testing on one platform for the simultaneous detection of AAb by cell-based and bead-based assays in one reaction environment. The multiplex analysis of antineutrophil cytoplasmic antibodies (ANCA) for the differential diagnosis of vasculitides will be discussed as a first application of this novel approach.
by Dr. Christina Fritz, Mandy Sowa and Dirk Roggenbuck
ANCA-associated vasculitis
Vasculitis is an inflammation affecting blood vessel walls and resulting in their damage, fibrinoid necrosis, tissue ischemia and necrosis, and finally vessel rupture with bleeding into the surrounding tissue [1, 2]. Due to etiological factors, systemic vasculitis is differentiated into primary and secondary vasculitis. Primary systemic vasculitis of particularly small vessels often has an autoimmune pathogenesis accompanied by the occurrence of ANCA [3,5-8]. Those so called ANCA-associated systemic vasculitides (AASV) comprise microscopic polyangiitis (MPA), eosinophilic granulomatosis with polyangiitis (EGPA or Churg-Strauss syndrome) or granulomatosis with polyangiitis (GPA or Wegener’s granulomatosis)[1, 2, 4]. In contrast, secondary vasculitis occurs in 5 – 10 % of patients with rheumatoid arthritis or with other autoimmune diseases (e.g., systemic lupus erythematosus [SLE], Sjögren’s syndrome). In addition, vasculitis can occur in patients suffering from infections such as HIV or hepatitis C.
In general, an acute AASV generally requires immunosuppressive treatment with high doses of cortisone. In severe cases, cyclophosphamide is recommended. Once remission is achieved, methotrexate, azathioprin, cotrimoxazol, leflunomid or mucophenolate mofetil are used as maintenance therapy.
Diagnosis of ANCA-associated vasculitis
According to the international consensus statement for the assessment of ANCA, IIF on ethanol-fixed human neutrophils (ethN) is followed by confirmation with antigen-specific molecular immunoassays [6-8]. IIF reveals two ANCA patterns sub-classifying ANCAs into cytoplasmic ANCA (cANCA) and perinuclear ANCA (pANCA). Regarding the autoantigenic target of ANCA, c and pANCA are directed against proteinase 3 (PR3) and myeloperoxidase (MPO), respectively. A positive cANCA pattern confirmed by the presence of PR3-ANCA is pathognomonic for GPA[5], whereas a positive pANCA pattern confirmed by MPO-ANCA is decisive for MPA and EGPA. Furthermore, the corresponding ANCA titres are strongly associated with activity of disease in patients suffering from GPA and MPA.
As a matter of fact, IIF is currently the only technique to provide a single reaction environment for the combined screening and confirmation of ANCA. Simultaneous detection of c and pANCA along with PR3- and MPO-ANCA would overcome time-consuming single parameter detection by different techniques [10].
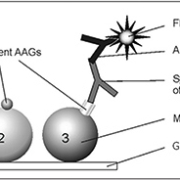
The use of multiplexing bead-based IIF assays for the simultaneous detection of single ANCA reactivities provides the ideal reaction environment to be combined with ethN-based ANCA testing. The corresponding principle is based on a covalent surface immobilization of MPO and PR3 on microbeads coded by size and fluorescence. The differentiation in size and/or intensity of a red fluorescence dye filling entirely each microbead population generates a novel reaction environment for parallel analyte analysis [11] (figure 1).
Combination of cell-based and microbead based ANCA assessment by CytoBead assay
The CytoBead assay is a unique combination of a conventional cell-based immunofluorescence assay with multiplexing microbead technology in one reaction environment. A newly designed microscopic glass slide with triple parted wells is employed to fix ethN in the middle compartment and PR3- as well as MPO-coated microbeads in the right-hand compartment of the slide (figure 2). Thus, anti-PR3 antibody positive sera show a positive cytoplasmic fluorescence on ethN and a green fluorescence halo on the surface of PR3-coated microbeads (9 µm). In contrast, anti-MPO antibody positive sera demonstrate a perinuclear fluorescence pattern on the immobilized ethN and a fluorescence halo on the surface of MPO-coated microbeads (15 µm) (figure 2). A reference microbead population (12 µm) is integrated for particle differentiation. This assay set offers the possibility of classical evaluation by a simple fluorescence microscope as well as automated analysis by interpretation systems like the AKLIDES®.
A recent clinical study with classical ANCA testing revealed a relative sensitivity and specificity of 98 % and 99.2 % for the novel CytoBead ANCA assay, respectively. Remarkably, the CytoBead ANCA assay showed a better discrimination of GPA and MPA patients in contrast to the classical anti-MPO and anti-PR3 ELISA. The detected cut-off values were determined on the basis of fluorescence intensity given in arbitrary units [AU] (personal communication).
Conclusion and future perspectives
The increasing demand for cost-effective autoimmune diagnostics requires new multiplexing technologies combining screening and confirmatory testing in one reaction environment. Thus, the novel CytoBead technology is a promising opportunity to accomplish this goal as demonstrated for the comprehensive assessment of ANCA. Automated digital immunofluorescence employed by recently established novel diagnostic interpretation system solutions such as Aklides even offers quantification and standardization of ANCA detection. The CytoBead technology provides an ideal reaction environment for the multiplexing of antinuclear antibody assessment and the simultaneous detection of celiac disease-specific antibodies.
References
1. Watt RA, Scott DG. Recent advances in classification and assessment of vasculitis. Best Pract Res Clin Rheumatol. 2009; 23: 429-443
2. Jeanette JC, Falk RJ. Small-vessel vasculitis. N Eng J Med. 1997; 337: 1512-23
3. Gross WL, Trabant A, Reinhold-Keller E. Diagnosis and evaluation of vasculitis. Rheumatology (Oxford). 2000; 39: 245-52
4. Waller R, Ahmed A, Patel I, Luqami R. Update on the classification of vasculitis. Best Pract Res Clin Rheumatol. 2013; 27: 3-17
5. Bosch X, Guilabert A, Font J: Antineutrophil cytoplasmic antibodies. Lancet 2006, 368:404-18
6. Jennette JC, Falk RJ, Bacon PA, Basu N, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CG, Lamprecht P, Langford CA, Lugmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DG, Specks U, Stone JH, Takahashi K, Watts RA: 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitis. Arthritis Rheum. 2013, 65:1-11
7. Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, Hagen EC, Hoffman GS, Hunder GG, Kallenberg CG: Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum 1994, 37:187-92
8. Savige JF, Gillis DF, Benson E, Davies DF, Esnault VF, Falk RJ, Hagen EC, Jayne D, Jennette JC, Paspaliaris B, Pollock W, Pusey C, Savage CO, Silvestrini R, van der Woude F, Wieslander J, Wiik A: International Consensus Statement on Testing and Reporting of Antineutrophil Cytoplasmic Antibodies (ANCA). Am J Clin Pathol 1999, 111:507-13
9. Merkel PA, Polisson RP, Chang Y, Skates SJ, Niles JL: Prevalence of antineutrophil cytoplasmic antibodies in a large inception cohort of patients with connective tissue disease. Ann. Intern. Med. 1997, 126;866
10. Choi HK, Liu S, Merkel, PA, Colditz GA, Niles Jl: Diagnostic performance of antineutrophil cytoplasmic antibody tests for idiopathic vasculitides: metaanalysis with a focus on antimyeloperoxidase antibodies. J. Rheumatol. 2001, 28:1584
11. Grossmann K, Roggenbuck D, Schröder C, Conrad K, Schierack P, Sack U: Multiplex Assessment of Non-Organ-Specific Autoantibodies with a Novel Microbead-Based Immunoassay. 2011, Cytometry Part A! 79A: 118”125
Author
Dr. Christina Fritz*, Mandy Sowa and Dirk Roggenbuck
Medipan GmbH, Ludwig-Erhard-Ring 3,
15827 Dahlewitz,
Germany
*Corresponding author
E-mail: c.fritz@medipan.de