TDM of levetiracetam and pregabalin: the need and the method
Therapeutic drug monitoring of anti-epileptic drugs has greatly advanced since the development of colorimetric assays for the measurement of phenytoin and phenobarbital in the mid-1950s. Today, not only have laboratory technology and assay development advanced, but so have the pharmaceutical agents available for the treatment of epilepsy disorders. However, under UK National Institute for Health and Clinical Excellence (NICE) Guidelines, therapeutic drug monitoring is still justified for newer anti-epileptic drugs like levetiracetam and pregabalin, for which we have developed quick and robust LC-MS/MS assays.
by Jonathan C. Clayton, Katherine Birch and Carrie A. Chadwick
Background
Therapeutic drug monitoring (TDM) is an important consideration in the treatment of epilepsy. It has long been known that a dose of a given drug may be effective in one patient but not in another [1]. This is of particular importance when too high a concentration of drug can have toxic effects, and too low a concentration has no therapeutic effect. Problems arise when, in different patients, a specific dosage leads to a therapeutically significant concentration in one, but could be ineffective or even toxic in another. Understanding the relationship between dosage and the concentration of the active drug at receptor sites has long been a topic for research [2], which has led to the development of assays to measure the plasma concentration of anti-epileptic drugs (AEDs). TDM of AEDs has advanced since colorimetric assays for phenytoin and phenobarbital were developed in the mid-1950s [3]. Older AEDs such as phenytoin and valproate have narrow therapeutic ranges (the plasma drug concentration range below which the drug may be ineffective and above which the patient may experience toxic effects). However, even the plasma concentration at which a given drug is effective may vary from individual to individual, depending on a number of factors known as pharmacokinetics [4]. Many newer AEDs, such as lamotrigine and topiramate do not have the narrow therapeutic range as seen with the older AEDs, however, TDM is still applicable [5]. Today both older AEDs such as phenytoin, phenobarbital and sodium valproate as well as newer AEDs such as lamotrigine and topiramate are subject to TDM [4]. This has led to the development of new assays for monitoring the serum concentration of these drugs. Methods include immunoassays such as enzyme multiplied immunoassay technique (EMIT) and cloned enzyme donor immunoassay (CEDIA), kinetic interaction of microparticles (KIMS) and chemiluminescent assays (CLIA) [6]. However, more liquid chromatography-tandem mass spectrometry (LC-MS/MS) assays are being developed for newer AEDs, which can detect a number of AEDs in a single assay [7].
Best Practice Guidelines for TDM published in 2008 [1], along with a review discussing TDM of the newer AEDs [8] have provided a rationale for developing methods for two second generation AEDs, levetiracetam and pregabalin. These drugs are becoming increasingly popular with levetiracetam being used as an adjunct for partial and generalized tonic–clonic seizures, and pregabalin used as an adjunct for partial seizures [9]. Pregabalin, and to a lesser extent levetiracetam, is also used in the treatment of non-epileptic disorders such as neuropathic pain [9]. The increasing popularity of these drugs with clinicians has led to an increasing demand for determination of plasma concentrations of these drugs. TDM is justified for determining compliance with treatment with either drug, but also for determining overdosing, and dosing in renal failure, of levetiracetam.
Here, we describe methods for the detection and quantification of levetiracetam or pregabalin in serum using ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). The methodology is identical for both levetiracetam and pregabalin and so, should demand for TDM of these drugs increase in the future, there is scope for them to be combined into one assay.
Materials and methods
Levetiracetam (1 mg/mL in MeOH) and pregabalin (1 mg/mL in MeOH) stock solutions, levetiracetam-D6 (100 µg/mL in MeOH) and pregabalin-D6 (100 µg/mL in MeOH) were purchased from Cerilliant (distributed by LGC Standards, Middlesex, UK). EQA materials used for accuracy assessment were kindly supplied by the LGC Heathcontrol EQA scheme. HPLC grade water and methanol were purchased from Sigma-Aldrich Ltd (Poole, Dorset, UK). All other chemicals were purchased from Sigma-Aldrich Ltd or VWR Ltd. ClinChek® Control Levels 1 and 2 were purchased from RECIPE (Munich, Germany).
Standards
Standard solutions were made by preparing serial dilutions of stock solution in PBS/BSA (phosphate buffered saline containing 0.5% bovine serum albumin) (137 mmol/L NaCl, 2.7 mmol/L KCl, 5.4 mmol/L Na2HPO4•7H2O, 1.8 mmol/L KH2PO4, 0.5% BSA). The standards were stored at –20°C until use.
Internal standards
Each internal standard was prepared to a final concentration of 10 mg/L in HPLC grade methanol containing 50 mmol/L ZnSO4∙7H2O. The internal standards were stored at room temperature until use.
Sample preparation
For assay purposes, standards, quality control (QC) and serum samples were prepared in an identical fashion. In a 96-well plate, 80 μL internal standard solution (in ZnSO4 in MeOH) are added to 20 μL sample followed by agitation and centrifugation. Eighty microlitres of H2O was then added to each well, the plate heat sealed, agitated and centrifuged.
Chromatography and mass spectrometry
Chromatography was performed on a Waters Acquity UPLC system equipped with a Waters Acquity UPLC BEH C18 1.7 μm 2.1 x 50 mm column. Mobile phase A consisted of 10 mmol/L ammonium acetate and mobile phase B consisted of MeOH.
A flow rate of 0.5 mL/min was maintained for the run time of 2.5 minutes. A linear gradient of mobile phase B from 2% to 50% was run between 0 and 1 minutes, followed by a constant concentration of 50% mobile phase B. Ninety-eight per cent mobile phase B was run from 1.75 to 2.5 minutes. The injection volume was 5 μL.
Mass spectrometric determination was carried out using a Waters TQD in ESI+ mode. The source temperature was 130 °C, desolvation temperature was 400 °C, cone gas flow was 50 L/hr and the desolvation gas flow was 800 L/hr. Targetlynx™ software was used to process the data and quantify the drugs in the standards, controls and patient samples.
Method validation
Validation of the assays was carried out according to Honour [10]. Precision and bias were determined by measuring QC samples over 5 batches with 5 samples in each batch. The coefficients of variance (CVs) were calculated for intra-batch and inter-batch precision. Bias was calculated from the nominal target values for each of the QC materials.
Accuracy was assessed using EQA materials from the LGC Heathcontrol AE1 Anti-epileptic drug EQA scheme.
Matrix effects were determined by running a water blank, extracted water and extracted drug-free serum against a background infusion of each drug.
The limit of blank (LOB) was determined by running 10 extracted water samples and was quantified as the highest concentration measured in the absence of analyte.
The lower limit of quantitation (LLOQ) was determined by spiking drug-free serum with known quantities of each drug, and was quantified as the lowest detectable concentration whose CV was <15% and bias <20%.
Specificity was determined by spiking PBS/BSA with high concentrations of six more commonly used AEDs (carbamazepine, carbamazepine epoxide, phenobarbital, phenytoin, primidone and sodium valproate.
Carry-over was determined by spiking drug-free serum with high concentrations of each drug, and analysing followed by drug-free serum.
Results
Chromatography and mass spectrometry
Levetiracetam and levetiracetam-D6 had a retention time of 0.88 minutes and the cycle time from injection to injection was 3 minutes. Pregabalin and pregabalin-D6 had a retention time of 0.82 minutes and the cycle time from injection to injection was 3 minutes. The chromatography profile is identical for both of the drugs. The profile produced clean, sharp peaks with no co-eluting elements. The quantification transition for levetiracetam was m/z 170.90>69.16 and the confirmation transition was m/z 170.90>98.17. For pregabalin, the quantification transition was m/z 159.90>55.12 and the confirmation transition was m/z 159.90>83.08. For the internal standards, levetiracetam-D6 had the transition m/z 177.00>132.00 and pregabalin-D6 had the transition m/z 166.10>102.90.
Method validation
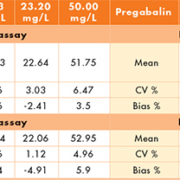
The intra- and inter-assay CVs are <8% for both drugs suggesting good precision of the assay. The inter- and intra-assay bias for levetiracetam was acceptable at <6%, while for pregabalin the inter- and intra-assay bias was <10% apart from the inter-assay bias at 10 mg/L (Table 1). External quality assessment materials were analysed as per patient samples. The results (Table 2) were compared with the target value supplied by LGC Heathcontrol, and with the returns of other laboratories using similar methods (LC-MS and LC-MS/MS) in order to determine the accuracy of the assay. Matrix effects were investigated using injections of drug-free serum, extracted water and blank water against a constant background infusion of each drug in methanol (50 mg/L levetiracetam, 25 mg/L pregabalin). No matrix effects are seen around the relevant retention times for either drug (Fig. 1). The LOB was quantified as the highest apparent analyte concentration in the absence of analyte. The LLOQ was quantified as the lowest level of analyte detectable whose CV was <15% and whose bias was <20% (Table 3). The methods for both levetiracetam and pregabalin showed no interference from any other commonly prescribed AEDs, with responses of ‘0’ to the interference samples from both methods. Blank serum samples and extracted water samples run immediately after samples containing either ~200 mg/L levetiracetam or 100 mg/L pregabalin gave responses of ‘0’, indicating no problems with carry-over.
Discussion
We have developed and validated LC-MS/MS assays for the quantification of levetiracetam and pregabalin in serum.
Two optimal transitions were identified for both drugs, thus providing a ‘quantifier’ transition and a ‘confirmation’ transition in order to increase confidence of identification owing to the risk of misidentification of analytes with the same molecular weights as the drugs of interest.
The chromatography method is identical for both levetiracetam and pregabalin, and with the two drugs having different retention times (0.88 and 0.82 minutes respectively), should there ever be a wish to combine these assays into one single run, this should be straightforward. Additionally, should assays for any other AEDs be developed, this chromatography method would be an appropriate starting point. Serum proteins are precipitated by the addition of ZnSO4 in methanol, which also aids the retained solubility of the drug. Following centrifugation, an equal volume of H2O is added so the drug is in 50 : 50 methanol/water. Following a further centrifugation, 5 µl of supernatant is injected onto the column. The method is quick and robust. The assay has acceptable precision and bias. All the EQA materials ran well within their acceptable ranges, close to the target value.
Other LC-MS/MS methods for the detection of levetiracetam [11, 12] and pregabalin [13] have been described, all of which have longer cycle times between injections, larger sample volume requirements, and, in some cases, have more complex sample preparation. The method described here benefits from being quick, with a simple sample preparation procedure.
Methods for the measurement of levetiracetam in saliva have been described [11] and it has been shown that there is good correlation between saliva, plasma and serum, meaning saliva would be a suitable alternative to serum [14]. To date, no such method has been described for pregabalin, but cases of pregabalin toxicity have been described which would advocate the development of further methods for the TDM of pregabalin [14].
The monitoring of levetiracetam and pregabalin is justified [1, 5] to monitor compliance and overdosing, and quick and robust methods for their measurement in serum have been described here. Further work could include development of assays for the measurement of these drugs in saliva, with comparison studies required.
References
1. Patsalos PN, Berry DJ, Bourgeois BF, Cloyd JC, Glauser TA, et al. Antiepileptic drugs—best practice guidelines for therapeutic drug monitoring: a position paper by the subcommission of therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia 2008; 49: 1239–1276.
2. Eadie MJ. Therapeutic drug monitoring—antiepileptic drugs. Br J Clin Pharmacol. 1998; 46: 185–193.
3. Theodore WH. Rational use of antiepileptic drug levels. Pharmac Ther. 1992; 54: 297–305.
4. Glauser TA, Pippenger CE. Controversies in blood-level monitoring: reexamining its role in the treatment of epilepsy. Epilepsia 2000; 41(Suppl. 8): S6–S15.
5. National Institute for Health and Clinical Excellence. The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care. Clinical guidelines 137. NICE 2012; http://guidance.nice.org.uk/CG137 (accessed 15 October 2013).
6. Aldaz A, Ferriols R, Aumente D, Calvo MV, Farre MR, et al. Pharmacokinetic monitoring of antiepileptic drugs. Farm Hosp. 2011; 35: 326–329.
7. Shibata M, Hashi S, Nakanishi H, Masuda S, Katsura T, Yano I. Detection of 22 antiepileptic drugs by ultra-performance liquid chromatography coupled with tandem mass spectrometry applicable to routine therapeutic drug monitoring. Biomed Chromatogr. 2012; 26: 1519–1528.
8. Krasowski MD. Therapeutic drug monitoring of the newer anti-epilepsy medications. Pharmaceuticals 2010; 3: 1909–1935.
9. Wahab, A. Difficulties in treatment and management of epilepsy and challenges in new drug development. Pharmaceuticals 2010; 3: 2090–2110.
10. Honour JW. Development and validation of a quantitative assay based on tandem mass spectrometry. Ann Clin Biochem. 2011; 48: 97–111.
11. Guo T, Oswald LM, Mendu DR, Soldin SJ. Determination of levetiracetam in human plasma/serum/saliva by liquid chromatography-electrospray tandem mass spectrometry. Clin Chim Acta 2007; 375: 115–118.
12. Blonk MI, van der Nagel BC, Smit LS, Mathot RA. Quantification of levetiracetam in plasma of neonates by ultra performance liquid chromatography-tandem mass spectrometry. J Chromatogr B. 2010; 878: 675–681.
13 Nirogi R, Kandikere V, Mudigonda K, Komarneni P, Aleti R. Liquid chromatography atmospheric pressure chemical ionization tandem mass spectrometry method for the quantification of pregabalin in human plasma. J Chromatogr B. 2009; 877: 3899–3906.
14. Patsalos PN, Berry DJ. Therapeutic drug monitoring of antiepileptic drugs by use of saliva. Ther Drug Monit. 2013; 35: 4–29.
The authors
Jonathan Clayton* MPhil, MSc; Katherine Birch DipRCPath; and Carrie Chadwick FRCPath
The Buxton Laboratories, The Walton Centre NHS Foundation Trust, Liverpool, UK
*Corresponding author
E-mail: Jonathan.clayton@nhs.net