NGAL as a biomarker of acute kidney injury
The diagnosis of acute kidney injury (AKI) is often hindered by the reliance on serum creatinine as a marker of kidney function, which can delay detection. Neutrophil gelatinase-associated lipocalin is a promising biomarker which increases within hours of kidney damage and could therefore improve the early diagnosis of AKI.
by Dr Ashley Garner
Clinical background
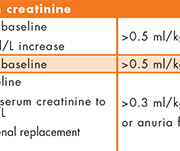
Acute kidney injury (AKI) is a common condition associated with significant morbidity and mortality. It is currently diagnosed using serum creatinine and urinary output as markers of kidney function, as defined in the recent KDIGO criteria [Fig. 1][1]. However, these are relatively late markers of AKI since they mainly reflect a decrease in glomerular filtration rate and the time required for serum creatinine to accumulate can delay diagnosis. Biomarkers that can detect structural injury to the kidney rather than a loss of function may allow better and earlier detection of AKI. Earlier diagnosis of AKI could facilitate earlier intervention, potentially reduce the risk of irreversible kidney damage and improve patient outcomes. Much research in recent years has therefore focused on the discovery of improved biomarkers for AKI and neutrophil gelatinase-associated lipocalin (NGAL) is one of the most promising candidates [2].
Pathophysiology of NGAL
NGAL is a small 25kDa protein which belongs to the superfamily of lipocalins. It is expressed in many cells including neutrophils, hepatocytes and renal tubular cells and is induced in response to pathological stimuli including infection, inflammation, ischemia and malignancy. NGAL has a functional role in the innate immune system as a bacteriostatic agent, depleting iron-binding siderophores and thereby preventing bacterial iron acquisition. The iron-binding properties of NGAL are also proposed to provide protection from oxidative stress. There is growing evidence that NGAL also acts as a growth factor in some tissues including renal epithelial cells where it modulates cell proliferation, differentiation and apoptosis and may provide protection against renal tubular damage in AKI [3].
Animal studies of AKI induced by ischemia or nephrotoxicity have shown that NGAL is one of the most upregulated proteins in the kidney and is detectable in the urine within 2–3 h. It has been reported that urine NGAL concentrations increase 25–100 fold and plasma NGAL increases 7–16 fold following AKI. Unlike serum creatinine, NGAL is not increased when there is impaired glomerular filtration without renal tubular damage, often termed ‘pre-renal’ uraemia.
Low plasma concentrations of NGAL are found in health as it is expressed at a low constant rate from various tissues. NGAL is then freely filtered at the kidney and the majority is reabsorbed in the proximal tubule, resulting in low NGAL concentrations in the urine. Following AKI, NGAL is greatly upregulated in the cells lining the ascending loop of Henle and collecting ducts of the kidney and is then excreted in the urine. The origin of the increase in plasma NGAL following AKI is less clear and there is evidence to suggest that NGAL expression is increased in other organs such as the lungs and liver following kidney injury.
Since NGAL can be produced by different tissues in response to various stimuli, it is not specific to AKI. Other common conditions that can cause elevated NGAL, and therefore complicate the interpretation of results, include sepsis, heart failure, chronic kidney disease (CKD), malignancy and urinary tract infections.
NGAL assays
Commercial CE marked assays are available for measuring NGAL in plasma, whole blood and urine. It is not clear from the literature whether any of these sample types are preferred or have better diagnostic performance but there are limiting factors for each that may require consideration. Plasma and whole blood samples are invasive and may be contaminated by haemolysis releasing NGAL from neutrophils. Urine NGAL may theoretically be more sensitive for AKI due to greater induction in renal tubular cells but can be falsely elevated in urinary tract infections due to leukocyturia and it is still unclear whether the NGAL should be corrected for urine concentration effects or whether this is unnecessary or even misleading in AKI. Although non-invasive, urine samples may be more difficult to obtain especially at specific time points or if the patient has reduced urine output.
NGAL exists in monomeric, dimeric and heterodimeric or complexed forms. It has been reported that the monomer is the predominant form produced by renal tubular cells and the homodimer is predominantly released by neutrophils. The relevance of these different forms of NGAL will depend on the extent to which NGAL assays detect them and the sample type used. Even though the monomer form may be most relevant for urine NGAL the origin of plasma NGAL in AKI is less clear and may therefore include the other forms. This variation in NGAL assays and sample types makes it difficult to directly compare study results and derive clinically relevant cut-off values. Standardization of NGAL assays using an internationally approved reference material would greatly improve this variation but this is not currently available and would require agreement on what forms of NGAL should be measured.
Research from large heterogeneous populations suggests that urine NGAL concentrations are dependent on gender, age and ethnicity. Biological variation for urine NGAL has also been reported to be as high as 84%. These factors will need to be taken into account when establishing reference intervals for NGAL although they may not be clinically significant if a cut-off value is used to diagnose AKI, especially if it greatly exceeds the expected reference intervals in health.
Clinical utility of NGAL in AKI
There is evidence that NGAL could be useful as an early diagnostic and prognostic biomarker for AKI. Many studies have demonstrated that NGAL rises 24–72 h before creatinine in patients with AKI and is associated with poorer outcomes. It is difficult to determine the diagnostic performance of NGAL for AKI in terms of clinical sensitivity and specificity however, due to the limitations of using serum creatinine as the gold standard comparator. For example, the rise in creatinine caused by pre-renal uraemia will not be associated with a raised NGAL. In addition, a multicentre pooled analysis of prospective studies has shown that patients who have raised NGAL without increases in serum creatinine are at increased risk of adverse outcomes and suggests these patients have a condition termed ‘subclinical AKI’ where there may be tubular damage without glomerular impairment [Fig. 2][4].
The majority of studies assessing NGAL testing in AKI have focused on specific patient populations at high risk of AKI: namely post-cardiac surgery, post contrast infusion, intensive care and emergency admissions.
The advantage of using NGAL in post-surgery or post-contrast patients is that the time of insult is known and therefore NGAL can be measured at set time points for the early detection of AKI and timely intervention. It is more difficult to determine the best application of NGAL in ICU patients in regard to the timing and frequency of tests and it is less clear whether earlier detection can improve outcomes in these patients frequently complicated by multi-organ failure. In the emergency admissions setting NGAL has fewer advantages over serum creatinine since early detection (within hours) is less likely to be applicable. Also NGAL, like creatinine, can be raised in CKD so may require multiple measurements to detect AKI but more patients are likely to have had a previous creatinine result than an NGAL result.
Although there is an abundance of observational studies showing that AKI can be detected earlier using NGAL compared to serum creatinine there is an absence of randomized clinical trials to demonstrate that using NGAL instead of current practice will improve patient outcomes or provide cost benefits. This is probably the biggest barrier to the adoption of NGAL testing in routine practice and better treatments and interventions may be required to overcome it. This would suggest that one of the most important roles for NGAL and earlier biomarkers of AKI is in the discovery and development of effective interventions and therapeutics.
Another consideration regarding interventions for AKI is that NGAL only detects renal tubular damage, it does not distinguish between different causes. However, effective treatments may require the underlying cause to be determined and therefore further biomarkers may be needed to differentiate between causative factors and indicate the most appropriate intervention.
Conclusion
A large number of clinical studies suggest that NGAL may provide an early diagnostic and prognostic biomarker for AKI. However, further randomized clinical trials comparing the use of NGAL to standard practice are required to show cost benefits or improvements in patient outcomes. It seems that even if biomarkers like NGAL enable us to detect AKI earlier, this alone may not be sufficient to improve patient care but hopefully they will facilitate the development of better interventions that will eventually lead to improved outcomes for patients with AKI.
References
1. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012; 2: 1–138.
2. ADQI Consensus on AKI Biomarkers and Cardiorenal Syndromes. Contrib Nephrol. Basel: Karger, 2013; 182: 13–29.
3. Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007; 18: 407–413.
4. Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, Krawczeski CD, Koyner JL, Murray P, Zappitelli M, Goldstein SL, Makris K, Ronco C, Martensson J, Martling CR, Venge P, Siew E, Ware LB, Ikizler TA, Mertens PR. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011; 57(17): 1752–1761.
The author
Ashley Garner PhD
Department of Blood Sciences,
Leeds Teaching Hospitals Trust, Leeds, UK
E-mail: Ashley.Garner@leedsth.nhs.uk