An electronic nose for asthma diagnosis
An electronic nose consists of an array of chemical sensors for the detection of volatile organic compounds and an algorithm for pattern recognition. Breath analysis with an electronic nose has a high diagnostic performance for atopic asthma that can be increased when combined with measurement of fractional exhaled nitric oxide.
by Dr Paolo Montuschi
Several volatile organic compounds (VOCs) have been identified in exhaled breath in healthy subjects and patients with respiratory disease by gas-chromatography/mass spectrometry (GC/MS) [1]. An electronic nose (e-nose) is an artificial system that generally consists of an array of chemical sensors for volatile detection and an algorithm for pattern recognition [2]. Several types of e-noses are available. An e-nose has been used for distinguishing between asthmatic and healthy subjects [3,4], between patients with asthma of different severity [3], between patients with lung cancer and healthy subjects [5], between patients with lung cancer and COPD [6], and between patients with asthma and COPD [7].
We compared the diagnostic performance of an e-nose with fractional exhaled nitric oxide (FENO), an independent method for assessing airway inflammation, and lung function testing in patients with asthma. We also investigated whether an e-nose could discriminate between asthmatic and healthy subjects and to establish the best sampling protocol (alveolar air vs oro-pharyngeal/airway air) for e-nose analysis. The results presented here are from a previously published study [4].
Methods
Study subjects
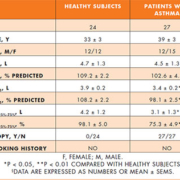
Twenty-four healthy subjects and 27 Caucasian patients with intermittent or mild persistent atopic asthma were studied [Table 1]. All asthmatic patients had a physician-based diagnosis of asthma, and the diagnosis and classification of asthma was based on clinical history, examination and pulmonary function parameters according to current guidelines [8]. Patients had intermittent asthma with symptoms equal to or less often than twice a week (step 1) or mild persistent asthma with symptoms more often than twice a week (step 2), forced expiratory volume in one second (FEV1) of 80% or greater of predicted value, and positive skin prick tests. Asthma patients were not taking any regular medication, but used inhaled short-acting β2-agonists as needed for symptom relief. Healthy subjects had no history of asthma and atopy, had negative skin prick tests and normal spirometry.
All subjects were never-smokers, had no upper respiratory tract infections in the previous 3 weeks, and were not being treated with corticosteroids or anti-inflammatory drugs for asthma in the previous 4 weeks.
Study design
The type of study was cross-sectional. Subjects attended on one occasion for clinical examination, FENO measurement, e-nose analysis, lung function tests, and skin prick testing. Informed consent was obtained from patients. The study was approved by the Ethics Committee of the Catholic University of the Sacred Heart, Rome, Italy.
Pulmonary function
Spirometry was performed with a Pony FX spirometer (Cosmed, Rome, Italy) and the best of three consecutive manoeuvres chosen.
Exhaled nitric oxide measurement
FENO was measured with the NIOX system (Aerocrine, Stockholm, Sweden) with a single breath on-line method at constant flow of 50 ml/sec according to American Thoracic Society guidelines [9].
Collection of exhaled breath
No food or drinks were allowed at least 2 hours prior to breath sampling. Two procedures for collecting exhaled breath were followed to study the differences between total exhaled breath and alveolar breath [4]. Subjects were asked to inhale to total lung capacity and to exhale into a mouthpiece connected to a Tedlar bag through a three-way valve [3]. In the first sampling procedure, the first 150 ml, considered as dead space volume, were collected into a separate Tedlar bag and discarded [Fig. 1a]. The remaining exhaled breath, principally derived from the alveolar compartment, was collected and immediately analysed with e-nose [4]. In the second sampling procedure, total exhaled breath was
collected [Fig. 1b] [4].
Electronic nose
A prototype e-nose (Libranose, University of Rome Tor Vergata, Italy), consisting of an array of eight quartz microbalance gas sensors coated by molecular films of metallo-porphyrins, was used [4]. E-nose responses are expressed as frequency changes for each sensor [Fig. 2] and then analysed by pattern recognition algorithms [2]. Ambient VOCs were subtracted from measures. Results were automatically adjusted for ambient VOCs.
Skin testing
Atopy was assessed by skin prick tests for common aeroallergens (Stallergenes, Antony, France).
Multivariate data analysis
Feed forward neural network was used to classify e-nose, FENO, spirometry data. A feed-forward neural network, a biologically derived classification model, is formed by a number of processing units (neurons), organised in layers. The datasets were divided into a training and a testing set. The first 27 measures collected were used for training and the remaining 24 measures for testing.
Statistical analysis
FENO values were expressed as medians and interquartile ranges (25th and 75th percentiles), whereas spirometry values were expressed as mean ±SEM. Unpaired t-test and Mann–Whitney U test were used for comparing groups for normally distributed and nonparametric data, respectively. Correlation was expressed as a Pearson coefficient and significance defined as a value of P<0.05.
Results
Electronic nose
The best results were obtained when e-nose analysis was performed on alveolar air as opposed to total exhaled breath [Table 2]. The diagnostic performance was determined in terms of the number of correct identifications of asthma diagnosis in the test dataset. Combination of e-nose analysis of alveolar air and FENO had the highest diagnostic performance for asthma (95.8%). The E-nose (87.5%) had a discriminating capacity that was higher than that of FENO (79.2%), spirometry (70.8%), combination of FENO and spirometry (83.3%), and combination of e-nose analysis of total exhaled breath and FENO (83.3%) [Fig. 3].
Exhaled nitric oxide
Median FENO values were higher in asthmatic patients than in healthy subjects [37. 6 (26.0–61.5) ppb vs 13.4 (10.0–19.9) ppb, P<0.0001, respectively].
Lung function tests
Both study groups had normal FEV1 values [Table 1]. Asthmatic patients had lower absolute (P = 0.032) and percentage of predicted FEV1 values (P = 0.004) than healthy subjects [Table 1]. Asthmatic patients had lower absolute (P = 0.003) and percentage of predicted forced expiratory flow between 25% and 75% of forced vital capacity (FEF25%–75%) (P = 0.002) than healthy subjects [Table 2].
Correlation between electronic nose, FeNO, and lung function tests
E-nose, FENO and lung function testing data were not correlated in either asthma or healthy control group.
Discussion
The original aspects of our study are:
1) the comparison between an e-nose and FENO, in addition to spirometry;
2) the comparison between total and alveolar exhaled air;
3) the analysis of data based on a neural network that included a training and a test analysis performed in two separate datasets for stringent quality control.
Our study indicates that an e-nose might be useful for asthma diagnosis, particularly in combination with FENO. Spirometry had the lowest diagnostic performance in line with a well-maintained lung function in patients with intermittent and persistent mild asthma. Our study confirms that FENO has a good diagnostic performance for asthma and suggests the possibility of using different non-invasive techniques for achieving a greater asthma diagnostic performance.
However, large powered studies are required to establish the diagnostic performance of e-nose, FENO and lung function testing in asthma patients. Ascertaining whether an e-nose could be used for screening of asthmatic patients requires large prospective studies. Also, the E-nose is not suitable for identifying and quantifying single breath VOCs, for which GC/MS is required.
Asthma is principally characterized by airway inflammation. It may seem surprising that the best results with the e-nose were obtained when collecting alveolar air rather than total exhaled breath which includes exhaled breath from the airways. This might reflect the contribution of oro-pharyngeal air which might introduce confounding factors making it e-nose analysis less reflective of what occurs within the respiratory system [10]. Moreover, the results of e-nose analysis of alveolar air could partially reflect the production of VOCs within the peripheral airways (mixed airways/alveolar air) due to significant inter-individual variability in dead space volume.
The lack of correlation between the e-nose results and those from FENO might indicate that these techniques reflect different aspects of airway inflammation. Formal studies to ascertain whether the e-nose could be used for assessing and monitoring airway inflammation in asthmatic patients are warranted. The E-nose is not suitable for ascertaining the cellular source of breath VOCs. Persistent airway inflammation can modify the metabolic pathways in patients with asthma. As patients included in our study were not on regular, anti-inflammatory drugs for asthma, we were unable to assess the effect of pharmacological treatment on breath VOCs, which requires controlled studies. Likewise, the effect of atopy on e-nose classification of asthma patients has to be addressed in future studies.
Validation of the classification model is essential. In our study, two different datasets for training and testing, obtained in different periods of time, were used. This way, the predictive capacity of the classification model is more suitable for a real life situation.
The E-nose analysis is a non-invasive technique that is potentially applicable to respiratory medicine. Several methodological issues including optimisation and standardisation of sample collection, transfer and storage of samples, use of calibration VOC mixtures, and qualitative and quantitative GC/MS analysis, have to be addressed.
In conclusion, an e-nose discriminates between asthma and healthy subjects and usage in combination with FENO increases the e-nose’s discriminatory ability. Large studies are required to establish the asthma diagnostic performance of e-nose. Whether this integrated non-invasive approach will translate into an early asthma diagnosis has still to be clarified.
Abbreviations
Abbreviations: FEF25%–75%, forced expiratory flow at 25% to 75% of forced vital capacity; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; GC/MS, gas chromatography/mass spectrometry; PEF, peak expiratory flow; VOC, volatile organic compound.
Acknowledgements
This study was supported by Merck Sharp and Dohme, and the Catholic University of the Sacred Heart.
References
1. Phillips M, Herrera J, et al. Variation in volatile organic compounds in the breath of normal humans. J Chromatogr B Biomed Sci Appl 1999; 729: 75–88.
2. Montuschi P, Mores N, et al. The electronic nose in respiratory medicine. Respiration (DOI: 10.1159/000340044, in press).
3. Dragonieri S, et al. An electronic nose in the discrimination of patients with asthma and controls. Allergy Clin Immunol. 2007; 120: 856–862.
4. Montuschi P, et al. Diagnostic performance of an electronic nose, fractional exhaled nitric oxide and lung function testing in asthma. Chest 2010; 137: 790–796.
5. Machado R, et al. Detection of lung cancer by sensor array analyses of exhaled breath. Am J Respir Care Med 2005; 171: 2186–1291.
6. Dragonieri S, et al. An electronic nose in the discrimination of patients with non-small cell lung cancer and COPD. Lung Cancer. 2009; 64: 166–170.
7. Fens N, et al: Exhaled breath profiling enables discrimination of chronic obstructive pulmonary disease and asthma. Am J Respir Crit Care Med 2009; 180: 1076–1082.
8. National Asthma Education and Prevention Program: Expert panel report III. Guidelines for the diagnosis and management of asthma. MD, Bethesda: National Heart, Lung, and Blood Institute, 2007; 1–61 (NIH publication no. 08-5847). Available at: www.nhlbi.nih.gov.
9. Recommendations for standardized procedures for the on-line and off-line measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children-1999: official statement of the American Thoracic Society 1999. Am J Respir Crit Care Med 1999; 160: 2104–2117.
10. van den Velde S, et al. Differences between alveolar air and mouth air. Anal Chem 2007; 79: 3425–3429.
The author
Paolo Montuschi, MD
Department of Pharmacology, Faculty of Medicine
Catholic University of the Sacred Heart
Largo F. Vito 1, 00168 Rome, Italy
E-mail: pmontuschi@rm.unicatt.it