A brief review of heparin-induced thrombocytopenia and diagnostic considerations
by Dr Christina C. Pierre and Dr Lindsay Bazydlo
Heparin-induced thrombocytopenia (HIT) is a hypercoagulable disorder that is mediated by antibodies against heparin complexed to platelet factor 4. HIT antibodies activate platelets, which generate a self-propagating, pro-coagulant state. A subset of patients with HIT develop thrombosis that can be limb- and/or life-threatening. This review provides a brief summary of HIT incidence, pathophysiology and clinical presentation. Clinical and laboratory diagnosis of HIT is described with a brief overview of HIT management. Lastly, current challenges and future perspectives on HIT diagnosis and management are discussed.
Background
Unfractionated heparin (UFH) and its low molecular weight derivatives are routinely utilized in therapeutic and prophylactic anticoagulation of surgical and medical patients. In spite of the availability of several newer oral and parenteral anticoagulants, heparin is likely to remain in clinical use for the near future owing to its favourable pharmacologic properties. Hemorrhagic events are the most common complications of heparin therapy; however, thrombotic complication can also occur in patients who develop heparin-induced thrombocytopenia (HIT). HIT is characterized as an adverse reaction to heparin that is mediated by platelet-activating antibodies against heparin/platelet factor 4 (PF-4) complexes. HIT is a hypercoagulable disorder with approximately one-half to one-third of patients with HIT developing thrombosis, which can be limb- or life-threatening. Consequently, it is critical that HIT is promptly diagnosed and alternative anticoagulation initiated.
Incidence of HIT
HIT incidence is estimated to be between 0.1% and 5% [1], depending on the type of heparin used, duration of exposure and patient population. HIT is less likely to occur following treatment with low molecular weight heparin (LMWH) compared to UFH [2]. One study reported a 79% reduction in the incidence of HIT following the implementation of a quality initiative where UFH was replaced with LMWH for prophylactic and therapeutic indications [3]. Exposure to heparin for greater than five days is a risk factor for developing HIT, with UFH conferring a risk ten times greater than LMWH [4]. Among surgical patients, the highest rates of HIT have been reported in cardiac, vascular and orthopedic surgery patients. High rates of HIT have also been observed in hemodialysis and trauma patients. Female surgical and hemodialysis patients are at higher risk for developing HIT compared to males [5, 6] and advanced age has also been associated with increased risk for HIT across both medical and surgical patients [6].
Pathophysiology of HIT
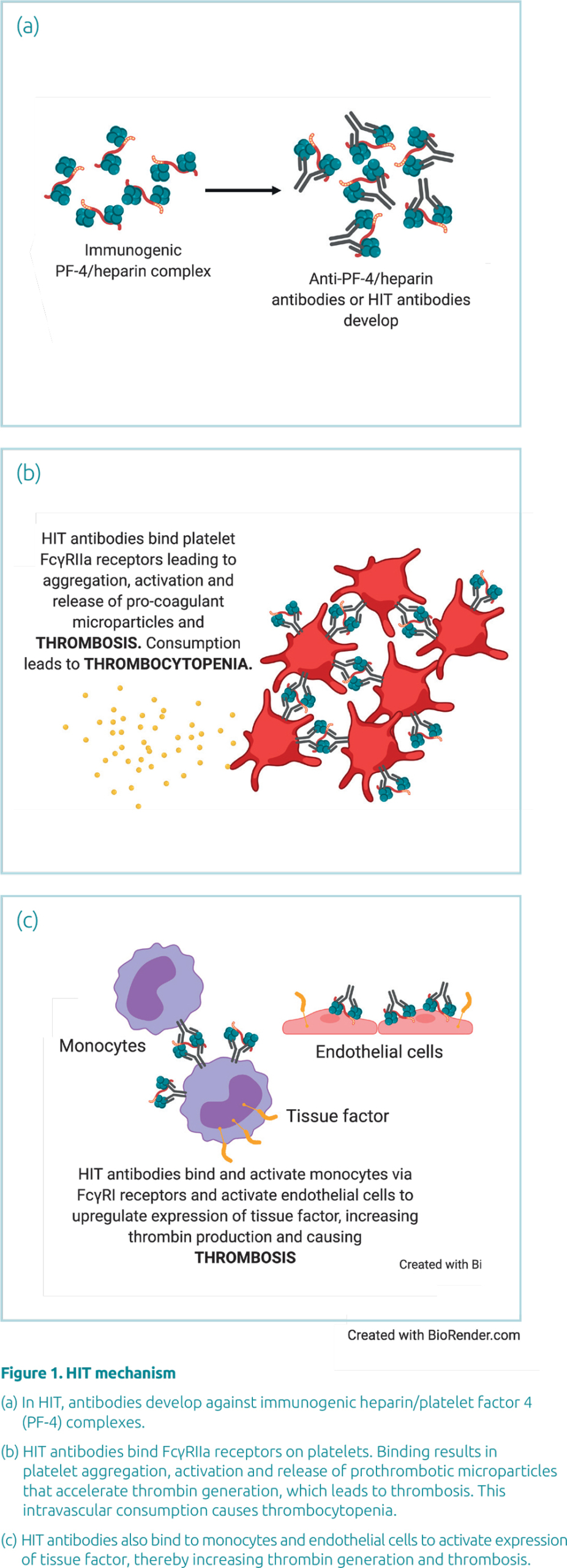
PF-4 is a positively charged chemokine that is released from the alpha granules of platelets upon platelet activation. PF-4 binds to negatively charged glycosaminoglycans on endothelial cell membranes, inhibiting antithrombin activity and thus promoting coagulation. Therapeutic heparin binds with high affinity to PF-4, displacing it from cellular and vascular binding sites into circulation. Heparin and PF-4 form large immunogenic complexes comprising of four PF-4 molecules bound to heparin. The interaction between heparin and PF-4 is based on electrostatic interactions. Consequently, differences in charge per molecule between UFH and LMWH are thought to account for the differences in immunogenicity and risk for developing HIT.
In heparin-naïve patients, antibodies become detectable at a median of 4 days post-heparin exposure, coincident with the maturation of precursor B cells into plasma cells that excrete large amounts of antibodies. Although IgG, IgM and IgA antibodies have been associated with HIT, IgG antibodies are thought to be the most clinically significant. IgG antibodies recognize and bind neo-epitopes formed through the interaction of heparin and PF-4 and activate monocytes and platelets via their FcγRI and FcγRIIa receptors respectively. Platelet activation leads to further release of PF-4, which propagates HIT pathophysiology, along with generation of thrombin, tissue factor, platelet-fibrin thrombi and pro-coagulant microparticles (Fig. 1).
Clinical presentation
The main clinical manifestation of HIT is thrombocytopenia with rapid post-exposure onset occurring in 30% of cases, onset within 5–10 days occurring in 60% of cases and delayed-onset occurring in the remainder [4]. The presence of residual antibodies from previous heparin exposure (within the past 100 days) is thought to be responsible for patients with rapid post-exposure onset of thrombocytopenia. The median platelet nadir in patients with HIT is about 55×109/L [4]. Thrombosis is the most severe complication of HIT. Venous thromboses are most common; however, arterial thrombosis and disseminated intravascular coagulation can also occur. Amputations occur in approximately 1–3% of patients that develop HIT with thrombosis and the mortality rate associated with HIT at approximately 5–10%.
HIT diagnosis
HIT is diagnosed using a three-pronged approach consisting of a clinical pre-test probability scoring system (the 4T score), an immunoassay that detects PF-4/heparin antibodies and a functional assay that determines whether detected antibodies are platelet activating. The 4T scoring system incorporates four clinical characteristics of HIT: magnitude of thrombocytopenia, timing of thrombocytopenia, thrombosis or other sequelae and ability to rule other causes of thrombocytopenia (Table 1) [7]. Accurate estimation of the 4T score can be challenging, particularly when incomplete data is available. Nevertheless, the negative predictive value of a low probability 4T score has been reported at 0.998, while the positive predictive values of high and intermediate 4T scores are 0.64 and 0.14 respectively [8]. The American Society of Hematology (ASH) recommends that heparin should be discontinued in favour of a non-heparin anticoagulant in patients with intermediate or high clinical probability for HIT, (4T score ≥4) and the patient should be tested for anti-PF4 antibodies [7].
Several different immunoassays are available for the detection of HIT antibodies that recognize different classes of antibodies and differ with respect to throughput and turnaround time. The most widely used immunoassays are enzyme-linked immunosorbent assay (ELISA)-based methods, which are available in both polyspecific and IgG-specific formats. These assays are time consuming, labour intensive and often batched. Other classes of assays have been developed that are random access to enable short turnaround times and a 24-hour service: particle gel immunoassay, particle immunofiltration assay, lateral flow immunoassay, latex agglutination assay, and chemiluminescent immunoassay. In a systematic review and meta-analysis of HIT immunoassays, the sensitivity and specificity of immunoassays for HIT varied widely depending on specificity (poly-specific vs IgG-specific), threshold for a positive result and whether a high-dose heparin confirmation step is performed [9].
ASH recommends that positive immunoassay results should be confirmed by a functional assay [7], with the serotonin release assay (SRA) being the gold standard. Generally, the optical density of ELISA-based methods or arbitrary concentration units of other immunoassay formats correlate with SRA positivity; that is, the higher the immunoassay result the more likely that the SRA will yield a positive result [10]. Consequently, ASH guidelines state that in patients with a high 4T score and a strongly positive immunoassay, a functional assay may not be necessary for diagnosis [7]. Serotonin is released from platelet dense granules upon platelet activation, and the percentage release of serotonin can be used to quantify the magnitude of platelet activation induced by HIT antibodies. In brief, platelet rich plasma (PRP) is prepared from pedigree blood donors whose platelets are known to release high amounts of serotonin. The PRP is incubated with 14C-serotonin and heat inactivated patient serum is then added, followed by a low and high dose of unfractionated heparin (for example, low dose: 0.1–0.3 U/mL and high dose: 100 U/mL). The SRA is considered negative if less than or equal to 20% release is observed. In order for the test to be positive, the low dose needs to display more than 20% release, while the high dose displays at least a 50% reduction compared to the low dose. High-dose heparin causes inhibition of platelet activation in the SRA, as it imbalances the stoichiometry required for heparin-antigen formation. Very few laboratories perform functional assays for HIT diagnosis, as the assays are lab-developed and labour-intensive. These assays offer high sensitivity and a higher specificity for HIT compared to immunoassays. False positive SRA results have been reported [10], and can be mitigated through reflexive testing algorithms in which only antibody-positive specimens are tested by SRA or the SRA and immunoassay testing is performed concurrently.
Management of patients with HIT
Once a HIT diagnosis is confirmed, the patient is labelled as having acute HIT. Management of acute HIT involves discontinuation of heparin and initiation of a non-heparin anticoagulant. Heparin discontinuation is not sufficient to eliminate the risk for thrombosis in patients with HIT, as the 30-day risk for thrombosis after heparin withdrawal is estimated to be between 19 and 52% [4]. HIT therapy should be individualized, depending on the type of patient, kidney and liver function, likelihood of additional procedures and bleeding risk. Direct thrombin inhibitors (bivalirudin, argatroban, dabigatran), indirect factor Xa inhibitors (danaparoid, fondaparinux) and direct factor Xa inhibitors (apixaban, rivaroxaban) have been recommended for management of acute HIT [7]. Complete platelet recovery is seen in approximately 65% of patients within one week of heparin cessation, although thrombotic risk remains high for 4 to 6 weeks after diagnosis. Patients may be monitored by HIT immunoassays to determine when antibodies are no longer present. The median time to HIT seronegativity is 85–90 days [11].
Current challenges and future considerations
Early diagnosis and intervention is critical in mitigating morbidity and mortality associated with HIT. As previously mentioned, calculation of the 4T score can be challenging, which is further compounded by the fact that thrombocytopenia occurs frequently in hospitalized, heparin-treated patients. Inaccurate estimation of the 4T score can lead to inappropriate HIT testing in patients with a low pre-test probability for HIT and an increase in the number of false positive results.
Another key challenge in HIT diagnosis is that only a small fraction of seropositive patients develops clinically significant HIT. Since functional assays are most often performed off-site, future immunoassays should focus on improved specificity for HIT. It is encouraging that the degree of immunoassay test positivity correlates with functional assay-positivity; however, further multicentre, assayspecific studies are required to evaluate the reliability of this measure alone or in combination with the 4T score in the prediction of HIT. Lastly, variation in the performance characteristics of the same functional assay format may exist between labs, since these assays are lab-developed. Since diagnosis hinges heavily on the functional assay result, it is critical that clinicians are familiar with the performance characteristics of the functional assay used at their institute and consider the entire clinical picture in making a diagnosis.
Interestingly, the coronavirus disease 2019 (COVID 19) is characterized by a high rate of thrombotic complications. Heparin thromboprophylaxis is used in certain subsets of COVID 19 patients and one study found an almost 10 fold higher occurrence of HIT during severe COVID-19 [12]. Furthermore, the incidence of HIT in ICU patients with severe COVID 19 was 8%. The pathophysiology of increased thrombosis risk in COVID 19 is poorly understood and further studies are needed to determine the cause of higher HIT incidence in these patients, as well as appropriate nonheparin anticoagulants for management.
Conclusion
Although a relatively rare complication, the morbidity and mortality associated with HIT warrants a high index of clinical suspicion in patients on heparin with otherwise unexplained thrombocytopenia. Early diagnosis and treatment can mitigate adverse outcomes associated with HIT. Laboratory testing is critical in HIT diagnosis, and it is important that clinicians understand the performance characteristics and limitations of HIT assays to enable appropriate management.
The authors
Christina C. Pierre1 PhD and Lindsay Bazydlo*2 PhD
- Department of Pathology, Penn Medicine Lancaster General Hospital, Lancaster, PA, USA
- Department of Pathology, University of Virginia School of Medicine, Charlottesville, VA, USA
*Corresponding author
E-mail: LAL2S@hscmail.mcc.virginia.edu
References
- Hogan M, Berger JS. Heparin-induced thrombocytopenia (HIT): Review of incidence, diagnosis, and management. Vasc Med 2020; 25(2): 160–173.
- Junqueira DR, Zorzela LM, Perini E. Unfractionated heparin versus low molecular weight heparins for avoiding heparin-induced thrombocytopenia in postoperative patients. Cochrane Database Syst Rev 2017 Apr 21; 4(4): CD007557.
- McGowan KE, Makari J, Diamantouros A, et al. Reducing the hospital burden of heparin-induced thrombocytopenia: impact of an avoid-heparin program. Blood 2016; 127(16): 1954–1959.
- Salter BS, Weiner MM, Trinh MA, et al. Heparin-induced thrombocytopenia: a comprehensive clinical review. J Am Coll Cardiol 2016; 67(21): 2519–2532.
- Warkentin TE, Sheppard JA, Sigouin CS, et al. Gender imbalance and risk factor interactions in heparin-induced thrombocytopenia. Blood 2006; 108(9): 2937–2941.
- Dhakal B, Kreuziger LB, Rein L, et al. Disease burden, complication rates, and health-care costs of heparin-induced thrombocytopenia in the USA: a population-based study. Lancet Haematol 2018; 5(5): e220-e231.
- Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv 2018; 2(22): 3360–3392.
- Cuker A, Gimotty PA, Crowther MA, Warkentin TE. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood 2012; 120(20): 4160–4167.
- Nagler M, Bachmann LM, ten Cate H, ten Cate-Hoek A. Diagnostic value of immunoassays for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood 2016; 127(5): 546–557.
- Warkentin TE, Arnold DM, Nazi I, Kelton JG. The platelet serotonin-release assay. Am J Hematol 2015; 90(6): 564–572.
- Arepally GM, Ortel TL. Heparin-induced thrombocytopenia. Annu Rev Med 2010; 61: 77–90.
- Daviet F, Guervilly C, Baldesi O, et al. Heparin-Induced Thrombocytopenia in Severe COVID-19. Circulation 2020; 142(19): 1875–1877.