ANCA-IIF: Still the screening method of choice
Systemic vasculitides are a group of inflammatory idiopathic clinical syndromes usually classified by the size of the vessels being affected. Among them, the small vessels vasculitides show clear associations with the presence in the patients sera of antibodies directed against cytoplasmic antigens of neutrophils (ANCA). Thus, circulating antibodies against neutrophil cytoplasm is the most characteristic feature of the serological profile in patients with autoimmune vasculitides, as Wegener’s granulomatosis, microscopic polyangiitis and Churg-Strauss syndrome, the so-called anti-neutrophil cytoplasmic antibody-associated systemic vasculitides, or ANCA-associated vasculitis (AAV). This group of diseases show a mean prevalence of 20 per million inhabitants, with a higher rate in the age group of individuals older than 70.
by Dr Petraki Munujos
There are typically two laboratory techniques for ANCA detection, indirect immunofluorescence (IIF) and enzyme-linked immunosorbent assay (ELISA), that are meant to be used in a combined form: the sera showing positive fluorescence in the IIF assay are tested by means of ELISA to confirm the specificity of the antibodies directed against either proteinase 3 (PR3) or myeloperoxidase (MPO).
The classical terms used to describe the fluorescent patterns observed in the IIF test on human neutrophil granulocytes, C-ANCA (cytoplasm) and P-ANCA (perinuclear), refer to antibodies directed to antigenic targets located in the primary granules of neutrophils, like PR3, MPO, lactoferrin, catalase, enolase, elastase or human lysosomal membrane glycoprotein (h-lamp2), among others. In addition, there are also the so-called atypical patterns due to other antigenic targets different from PR3 and MPO or to a combination of specifities. The following are the four fluorescent patterns that can be observed with IIF on human neutrophils [1]:
• C-ANCA, showing granular cytoplasmic neutrophil fluorescence with central interlobular accentuation. It corresponds in most of the cases to PR3 specificity.
• P-ANCA, with perinuclear neutrophil staining, often with nuclear extension, mostly corresponding to MPO specificity.
• ANCA-atypical or X-ANCA. The pattern is a mix of cytoplasmic and perinuclear rim-like fluorescence, and it is due to multiple specificities.
• C-ANCA-atypical. The cytoplasmic fluorescence is homogeneous, with no interlobular accentuation. Mainly due to the reaction of the antibodies with BPI (bactericidal/permeability-increasing protein).
However, it is important to keep in mind that the antigenic specificities corresponding to the fluorescent patterns observed are not clear, and that the assumption of C-ANCA being equivalent to PR3 and P-ANCA, to MPO is not correct. The positive results obtained by IIF must always be confirmed by specific ELISA tests.
A principle based on an artifact
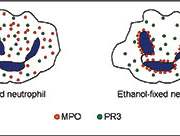
Both PR3 and MPO are lysosomal enzymes that can be found in azurophilic granules in the cytoplasm of human neutrophilic granulocytes. The ethanol fixation of the neutrophil makes the positively charged proteins from the cytoplasm migrate towards the negatively charged nuclear membrane [figure 1]. As a result of this treatment, myeloperoxidase molecules in ethanol-fixed neutrophils locate in the perinuclear area instead of the cytoplasm. This circumstance makes it possible to distinguish the antibodies reacting with MPO from those reacting with PR3, since differentiated stained structures can be observed through the microscope [figure 2].
Laboratory detection of ANCA
As mentioned before, a sample analysed for the presence of ANCA by indirect immunofluorescence can show up to 4 different staining patterns, but C-ANCA and P-ANCA are by far the most commonly reported type of results. Since the correspondence with a target antigen is not unique for any of the stainings, sera containing ANCA should be tested in ELISA at least for both PR3- and MPO-ANCA. While the C-ANCA patterns clearly stain cytoplasmic components of the neutrophil, the P-ANCA pattern may be due to either cytoplasmic or nuclear targets, since distinguishing a cytoplasmic perinuclear staining from an homogeneous nuclear staining is unlikely. Consequently, sera containing P-ANCA should be further tested to discriminate those due to antinuclear antibodies (ANA) from those with antibodies directed to cytoplasmic antigens like myeloperoxidase. There have been classically two approaches for this purpose, consisting in testing the P-ANCA sera either to confirm the perinuclear localization of the antigenic target or to detect the presence of antibodies directed to nuclear structures like DNA or Histones. The first option is based on the use of neutrophils that have not been fixed with ethanol, therefore, the myeloperoxidase molecules have not migrated towards the perinuclear region of the cell. These neutrophil slides are fixed with formalin instead, allowing the myeloperoxidase to remain in its original cytoplasmic location. As a result, a serum containing anti-myeloperoxidase antibodies will show P-ANCA staining pattern on ethanol-fixed neutrophils and C-ANCA staining pattern on formalin-fixed neutrophils [Figure 3]. On the other hand, a serum containing ANA will show P-ANCA staining pattern on ethanol-fixed neutrophils and nuclear homogeneous staining pattern, often of weak intensity, or negative staining on formalin-fixed neutrophils [Figure 3].
The second approach to discriminate true P-ANCA sera from those containing antinuclear antibodies consists in testing for the presence of such antibodies, basically on HEp2 cells [2]. This option may represent an additional cost, since the result only reveals the presence or absence of ANA but does not show direct evidences of the presence of antibodies directed against the cytoplasm of neutrophil. Some laboratories test in parallel both parameters, ANA and ANCA in a separate analysis. There have been some attempts to combine both ANA and ANCA tests in the same substrate, i.e., by mixing neutrophils and lymphocytes in the cellular preparation, but with controversial results due presumably to some incompatibility between the respective manufacturing procedures.
Besides the two typical patterns C-ANCA and P-ANCA, an unusual mixture of both staining patterns can be found, called atypical, or X-ANCA. On the other hand, not all myeloperoxidase molecules migrate to the perinuclear rim after ethanol fixation, and a “hybrid pattern” of both cytoplasmic and perinuclear staining can be observed and due to incomplete relocation of the antigen. Other antigens such as lactoferrin, elastase, BPI, cathepsin G, lysozyme, h-lamp-2 and enolase usually show X-ANCA pattern.
X-ANCA pattern (or atypical P-ANCA) is the result of the autoantibody binding mainly to the proteins cathepsin G, lysozyme and lactoferrin. These autoantibodies are not found in patients with Wegener’s granulomatosis, microscopic polyangiitis or Churg-Strauss syndrome, but are more often associated with inflammatory bowel diseases. Such proteins are released from the neutrophil specific granules and locate in the periphery of the nuclei after ethanol, acetone or formalin treatment [3]. It is remarkable that the mentioned antigens do not show a different distribution within the cell as a result of the fixative used, contrary to what happens with MPO. This fact helps to distinguish the antibodies reacting against MPO (P-ANCA with ethanol, C-ANCA with formalin) from those reacting to cathepsin G, lysozyme or lactoferrin (P-ANCA regardless the fixative used). (Figure 4). P-ANCA can be distinguished from atypical P-ANCA by confocal microscopy in that the atypical p-ANCA show multiple intranuclear fluorescent invaginations of the multilobulated neutrophil nuclear envelope. However, this feature is very difficult to observe with a conventional microscope [4].
As mentioned, the perinuclear nature of some ANCA reactions is an artifact due to the ethanol fixation of neutrophils, causing a redistribution of the cytoplasmic granules around the nucleus. However, the atypical P-ANCA or X-ANCA reaction observed in patients with inflammatory bowel diseases like ulcerative colitis (UC) has been found not to be similarly affected, since the corresponding antigens have a nuclear localization. The comparison of IIF patterns of sera tested on different fixed cells may be useful to distinguish vasculitis-related P-ANCA versus ANA and vasculitis-related P-ANCA versus UC-related P-ANCA. On the other hand, methanol fixation seems to provide a brighter staining on X-ANCA patterns, as compared with ethanol-fixed neutrophils. However, some MPO positive sera become negative with this fixative, as compared with ethanol fixation, whereas no such effect is observed with PR3-ANCA and ANA positive sera [5].
ANCA are much more common in UC than in Crohn’s disease (CrD). ANCA testing alone does not distinguish between the two diseases, but the combination of ANCA test by IIF and anti–Saccharomyces cerevisiae antibody (ASCA) test by ELISA often aids their differentiation. Patients with UC are more likely to have P-ANCA–positive and ASCA-negative results, and those with CrD are more likely to have P-ANCA–negative and ASCA-positive results. Also, UC patients with moderate and severe disease usually have higher titres of ANCA than patients in remission or with mild disease [6]. Due to the difficulties in distinguishing X-ANCA pattern from P-ANCA with the microscope, the determination of the final ANCA autoantigen specificity is often performed by means of ELISA assays using the corresponding specific antigen as microplate covering reagent.
Harmonization in ANCA testing
As a semi quantitative technique, indirect immunofluorescence results are expressed as low, moderate and strong positive, or negative, based on the analyst’s skills and interpretation. More than titrating the strong positive samples, what the international committees of experts recommend is to run additional tests to check the specificity by means of ELISA [7]. Issues like the initial dilution, the choice of substrates or the sequence of tests to be performed are key aspects to further advance towards a better understanding of the laboratory data.
One of the goals of any organization devoted to standardization in the clinical laboratory is to establish harmonized testing algorithms. The European Autoantibody Standardization Initiative (EASI) has promoted several studies in that sense. Internationally standardized algorithms used by the different laboratories would contribute to the rational use of laboratory tests and would lead to a more efficient usage of the laboratory tests. Figure 5 shows the algorithm proposed by Conrad and colleagues faced with the suspicion of a systemic ANCA-associated vasculitis.
The International Consensus Statement advocates screening by IIF and confirmation of IIF positivity in PR3-ANCA and MPO-ANCA ELISAs [8], and this is a good example of the efforts addressed towards harmonization. However, many others aspects regarding not only the testing, but the manufacturing processes of IVD immunofluorescence reagents still need to be taken care of in terms of standardization.
References
1. Savige J, et al. Kidney International 2000; Vol. 57: 846–862.
2. Lochman I, et al. Autoimmunity Reviews 2011; 10:295-298.
3. Billing P, et al. American Jouirnal of Pathology 1995; 147(4): 979-987.
4. Terjung B, et al. Clin Exp Immunol 2001; 126: 37-46
5. Radice A, et al. Clinical and Experimental Rheumatology 2000; 18: 707-712.
6. Dobric S, et al. Clin Chem Lab Med 2012; 50(3): 503–509.
7. Luxton G, et al. Nephrology 2008; 13: S17–S23.
8. Savige J, et al. Am J Clin Pathol 2003; 120: 312-318.
9. Conrad K, et al. Autoantibodies in Organ Specific Autoimmune Diseases. A Diagnostic Reference. Autoantigens, Autoantibodies, Autoimmunity vol. 8 – 2011. PABST Science Publishers.
The author
Petraki Munujos, PhD
Biosystems SA
Barcelona, Spain