Anti-glycolipid antibodies: their role in neurological disease and their detection
The relationship between anti-glycolipid antibodies and peripheral neuropathies has for many years been studied by laboratory investigations because of the antibody-phenotype associations identified. This has led to the discovery of a number of peripheral nerve conditions affected by the presence of anti-glycolipid antibodies. This article describes some of the conditions associated with anti-glycolipid antibodies, and the techniques available to detect them in a specialist laboratory setting.
by Carrie A. Chadwick
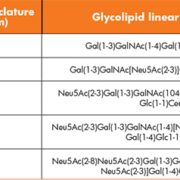
Glycolipid nomenclature
The name ganglioside was first applied by the German scientist Ernst Klenk in 1942 to lipids newly isolated from ganglion cells of brain [1]. Gangliosides are also referred to as glycolipids; they are glycosyl derivatives of lipids such as acylglycerols and ceramides. They are part of a larger family of substances known as glycoconjugates, which also includes glycoproteins, glycopeptides, peptidoglycans and lipopolysaccharides.
The official nomenclature for glycolipids is that of the Joint Commission on Biochemical Nomenclature (JCBN) formed from the International Union of Pure and Applied Chemistry (IUPAC) and the International Union of Biochemistry and Molecular Biology (IUBMB). The current recommendations are those published in 1997 [2].
The Svennerholm nomenclature [3] is simpler and relies on the fact that glycolipids are known to be oligoglycosylceramides containing N-acetylneuraminic acid (sialic acid or ‘NANA’ or ‘SA’ or Neu5Ac) residues joined via glycosidic linkages to one or more of the monosaccharide units. As a result, the polar head groups of the lipids carry a net negative charge at pH 7.0 and they are acidic. Glycolipids are also considered to be amphiphilic, as they have a polar hydrophilic oligosaccharide head, coupled to a non-polar lipophilic tail. They are, therefore, soluble in both organic solvents and water. Svennerholm nomenclature is that encountered most often in the literature; a short-hand nomenclature system in which M, D and T refer to mono, di, and trisialogangliosides, respectively, and the numbers 1, 2, 3, etc. refer to the order of migration of the ganglioside in thin layer chromatography (TLC). For example the order of migration of monosialogangliosides is GM3>GM2>GM1. To indicate variations within the basic structures, further designations are added, e.g. GM1a, GD1b, etc. Table 1 shows the application of the Svennerholm nomenclature to the common gangliosides.
Glycolipids are not simple linear glycan chains; they are complex three dimensional structures. These conformational structures are closely related to their functions in biological systems.
Glycolipids are abundant in the brain in which they can amount to 6% of the weight of lipids, rising to 10–12% of the total lipid content (20–25% of the outer layer) of isolated neuronal membranes. They are ubiquitous and occur in low levels (1–2%) in all animal tissues, where, like the neutral oligoglycosphingolipids, they are thought to be concentrated in ‘rafts’ in the plasma membrane. The location of the glycolipids within these ‘rafts’ enables them to affect receptor function and signal transduction [4, 5]. The pathogenic role of anti-glycolipid antibodies depends upon how they affect the number and distribution of the target glycolipids and how they also interfere with the neuronal function of the target glycolipid.
Guillain–Barré syndrome and variant conditions
Guillain–Barré syndrome (GBS) is a rare and serious condition affecting the peripheral nervous system. The exact cause of GBS is unknown. It was first described by Guillain, Barré and Ströhl in 1916 [6] as a symmetrical, rapidly evolving flaccid paralysis and areflexia. It has since been sub-classified based on the variation of the disease being either axonal or de-myelinating [7]. Approximately 60% of people with GBS acquire the condition after having either a viral or bacterial infection. The infection is believed to trigger the immune system into attacking the nerve roots and peripheral nerves. The symptoms of GBS usually develop 2–4 weeks after a minor infection, such as a cold, sore throat or gastroenteritis. Symptoms usually start in the feet and hands before spreading to the arms and legs. Patients usually complain of pain, tingling and numbness with progressive muscular weakness and co-ordination problems with feeling unsteady when walking.
Anti-glycolipid antibodies were first identified in cases of GBS in 1988 [8]; there were a number of publications at this time on the presence of anti-glycolipid antibodies in chronic demyelinating polyneuropathies and multifocal neuropathies. It was identified that there were many interesting parallels with these conditions and GBS. From the research carried out it has become apparent that not all patients with GBS will have identifiable anti-glycolipid antibodies. The association of specific anti-glycolipid antibodies with defined clinical GBS including the variants is relatively weak and there is a lack of consistency. A clear and constant association between anti-GQ1b/GT1a antibodies has been made with Miller Fisher syndrome (MFS); the same cannot be said for other GBS variant diseases, e.g. acute motor axonal neuropathy (AMAN) [7].
Acute inflammatory demyelinating polyneuropathy (AIDP) is the most common variant of GBS presenting in Europe and North America, and it is described as a demyelinating variant with some lymphocytic infiltration [8]. Patients present with flaccid paralysis, areflexia and occasionally sensory loss [7].
Miller Fisher syndrome (MFS) is a rare syndrome with an estimated annual incidence of 0.09 per 100 000; it is the most common variant of GBS, accounting for 5–10 % of all GBS cases [7]. Patients present with acute onset of opthalmoplegia, ataxia and areflexia [10]. The anti-GQ1b antibody is present in 90% of cases of MFS; it is a highly specific finding as the anti-GQ1b IgG antibodies are not detectable in normal or other disease control groups [7]. It is most likely that the anti-GQ1b antibodies cross react with GT1a glycolipid as they are structurally similar [11].
Table 2 lists some of the clinical syndromes identified based on the antibody–phenotype associations.
It is important to note that the anti-glycolipid antibody assays from which much of the published literature are based are fraught with a number of issues including inter-laboratory variation due to a variety of screening protocols being available for their detection and lack of standardization of the anti-glycolipid antibodies in use [7, 12]. Epidemiological factors also influence the pattern of anti-glycolipid antibodies identified, due to the prevalence of preceding infections, such as Campylobacter jejuni, determining the subtypes of GBS in various geographic locations. A final consideration is the impact of the individual’s and/or the population’s genetic and environmental background and the impact this has on the immune response in the presence of glycolipid epitopes [7].
Analytical methods
Screening enzyme-linked immunosorbent assay (ELISA)
Screening for antibodies is performed using an ELISA in antibody detection mode. Simply, individual glycolipids are dissolved in polar organic solvents, typically methanol and ethanol, and added to individual wells of a polystyrene 96 well ELISA plate. The solvent is evaporated leaving the glycolipid with an electrostatic attachment to the plastic. A bovine serum albumin blocking agent is added to each well for 30 minutes and then discarded. The patient sample is then incubated and any antibodies to the glycolipid will bind. The wells are washed and then incubated with a mixture of goat anti-human IgG and goat anti-human IgM antibodies. Both of these detector antibodies are conjugated with horse radish peroxidase (HRP), and are in the form of F(ab)2 fragments, i.e. the Fc chain has been removed. This is done to reduce the risk of non-specific interaction, which commonly occurs between interspecies heavy chains. After a further incubation and wash, the wells are incubated with the chromogen tetramethylbenzidine (TMB) and hydrogen peroxide, the enzyme substrate. Enzyme activity produces a colour change that is measured and the amount of colour produced is proportional to the amount of antibody activity present.
Note that the screening test is intended to detect both IgG and IgM antibodies simultaneously, hence the use of a mixed detector antibody. If a positive result is found, it is then necessary to retest the sample individually for IgG and IgM antibodies. That is the purpose of the antibody titration outlined below.
It is not possible to mix glycolipids together for testing as this increases non-specific binding and complicates analysis and interpretation. Paradoxically, recent work suggests that in some patients, antibodies against some pairs of glycolipids show greater activity than against the individual component glycolipids [13, 14].
A significant source of error in the screening ELISA is the phenomenon of heterophile antibodies. Heterophile antibodies are endogenous antibodies present in the blood that react against proteins of other species. Although most commonly heterophile antibodies are anti-antibodies, they may also recognize other blood proteins, such as bovine serum albumin, which is commonly used as a blocking agent in antibody ELISAs [15]. There is extensive literature available on heterophile antibodies and the techniques used to try and minimize their influence. In the screening assay the biggest risk of error is due to anti-bovine serum albumin (BSA) heterophile activity. It is usually possible to circumvent the problem of anti-BSA heterophile activity by re-testing using an alternate blocking agent, typically human albumin.
Titrating antibody activity
When the screening test is positive, the sample is re-tested against the positive glycolipid, using IgG and IgM antibodies separately. This stage is usually coupled with testing serial dilutions of the sample to enable the titre of the antibody to be determined.
Isolation of glycolipids and high performance thin layer chromatography (HPTLC)
The major disadvantage of the screening/titration ELISA methods is that they only allow us to test for antibodies against a limited number of glycolipids. The glycolipids in our screening ELISA are the main glycolipid epitopes most commonly found in association with disease. This may be a reflection of the bias in the screening assay commonly applied around the world. At least 188 unique glycolipids have been described in nature. Testing for antibodies against all of these glycolipids would be a monumental task. Nevertheless, there are reports of antibodies against glycolipids that are not represented within the screening ELISA.
One approach to addressing this issue is to isolate glycolipids from mammalian brain and then separate them by high performance thin layer chromatography (HPTLC). Once separated, the glycolipids can be fixed in place by spraying the plate with plasticizer. The TLC plate can then be cut into strips and used for testing patient samples, similarly to protein immunoblots. This method, however, is rather labour intensive and is not routinely performed in our laboratory.
Combinatorial glycoarray
The ability to measure combinations and complexes of anti-glycolipid antibodies is now possible with the development of a novel technique known as combinatorial glycoarray [14, 16]. This is a semi-automated system, which allows patient samples to be tested against a large number of glycolipids and their complexes simultaneously. A TLC automatic sampler prints reproducible arrays of glycolipid complexes onto polyvinylidene difluoride membranes, which are attached to glass slides. They are left to dry overnight and, once dry, the lectin of interest is probed onto the glycoarray. A chemiluminescent reaction driven by horse radish peroxidase-linked secondary antibody enables detection on a radiographic film and quantification is then determined using image analysis software [16]. This technique has demonstrated good concordance with the ELISA technique and it has also shown enhanced detection of anti-glycolipid antibodies in patients with GBS [17].
With the advancement in glycoarray technology and the benefit of being able to screen against isolated and complexed anti-glycolipid antigens simultaneously; it is clear that specialist centres should be working towards employing this technology routinely to support neurologists with the investigation and diagnosis of inflammatory neuropathies.
Acknowledgement
I would like to acknowledge Dr Geoffrey Keir, Consultant Clinical Scientist for his unwavering support and enthusiasm.
References
1. Klenk E. Uber die Ganglioside des Gehirns bei der infantilen amaurotischen idiotie vom Typ Tay-Sachs. Eur J Inorg Chem. 1942; 75: 1427–2110 (in German).
2. Chester MA. Nomenclature of glycolipids (IUPAC recommendations). Pure Appl Chem. 1997; 69: 2475–2487
3. Svennerholm L. The gangliosides. J Lipid Res. 1964; 5: 145–155
4. Simons K, Ikonen E. Functional rafts in cell membranes. Nature 1997; 387: 569–572.
5. Stoffel W, Bosio A. Myelin glycolipids and their functions. Curr Opin Neurobiol. 1997; 7: 654–661.
6. Guillain G, Barré JA, Ströhl A. Sur un syndrome de radiculo-névrite avec hyperalbuminose du liquid céphalo-rachidien sans réaction cellulaire: remarques sur les caractères cliniques et graphiques des réflexes tendineux. Bill Soc Med Hop Paris 1916; 40: 1462–1470 (in French).
7. Willison HJ, Yuki N. Peripheral neuropathies and anti-glycolipid antibodies. Brain 2002; 125: 2591–2625.
8. Ilyas AA, Willison HJ, Quarkes RH, Jungalwala FB, Cornblkath DR, Trapp BD, Griffin DE, Griffin JW, McKhann GM. Serum antibodies to gangliosides in Guillain-Barré syndrome. Ann Neurol 1988; 23: 440–447.
9. Asbury AK, Arnason BG, Adams RD. The inflammatory lesion in idiopathic polyneuritis: its role in pathogenesis. Medicine 1969; 48:173–215.
10. Fisher M. An unusual variant of acute idiopathic polyneuritis: syndrome of opthalmoplegia, ataxia and areflexia. N Engl J Med. 1956; 255: 57–65.
11. Chiba A, Kusunoki S, Obata H, Machinami R, Kanazawa I. Ganglioside composition of the human cranial nerves, with specific reference to pathophysiology of Miller Fisher syndrome. Brain Res. 1997; 745: 32–36.
12. Willison HJ, Veitch J, Swan AV, Baumann N, Comi G, Gregson NA, Illa I, Jacobs BC, Zielasek J, Hughes RAC. Inter-laboratory validation of an ELISA for the determination of serum anti-ganglioside antibodies. Eur J Neurol. 1999; 6: 71–77.
13. Kaida K, Sonoo M, Ogawa G, Kamakura K, Ueda-Sada M, Arita M, Motoyoshi K, Kusunoki S. GM1/GalNAc-GD1a complex: A target for pure motor Guillain-Barré syndrome. Neurology 2008; 71:1683–1690.
14. Goodfellow JA, Willison HJ. Antiganglioside, antiganglioside-complex, and anti-glycolipid-complex antibodies in immune mediated neuropathies. Curr Opin Neurol. 2016; 29: 572–580.
15. Ravindranath MH, Muthugounder S, Saravanan TS, Presser N, Morton DL. Human antiganglioside autoantibodies: validation of ELISA. Ann N Y Acad Sci. 2005; 1050: 229–242.
16. Rinaldi S, Brennan KM, Willison HJ. Combinatorial glycoarray. Methods Mol Biol. 2012; 808: 413–423.
17. Delmont E, Robb H, Davidson A, Halstead S, Yao D, Meehan GR, Willison H. Prospective study comparing enzyme-linked immunosorbent assay and glycoarray assay to detect antiglycolipid antibodies in a routine diagnostic neuroimmunology laboratory setting. Clin Exp Neuroimmunnol. 2015; 6: 175–182.
The author
Carrie A. Chadwick BSc, MSc (Hons), FRCPath
The Neuroscience Laboratories, The Walton Centre NHS Foundation Trust, Liverpool, UK
E-mail: Carrie.chadwick@thewaltoncentre.nhs.uk