Anti-parietal cell antibodies
The anti-parietal cell antibodies show one of the most distinctive fluorescent patterns in the autoantibody screening by indirect immunofluorescence. Although these antibodies react with a well known target antigen (H+/K+ ATPase) solely present in the parietal cells of the gastric gland, the use of combined tissue sections in the same reaction well is highly recommended as an aid to avoid confusions with the more frequently found anti-mitochondrial antibodies.
by Dr Petraki Munujos
Autoantibody
Anti-parietal cell antibodies (APCA) is a serological marker used for the diagnosis of pernicious anemia and type A chronic gastritis. APCA belong to the IgG class, although IgA and IgM isotypes may also be detected in patients with chronic atrophic gastritis [1].
Target antigen
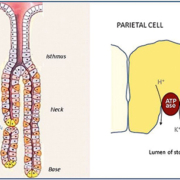
The major molecular targets of anti-parietal cell antibodies are the alpha- and the beta-subunits of the gastric proton pump H+/K+ ATPase in human autoimmune gastritis. The cells containing the antigen are located in the gastric gland (figure 1) and are responsible for hydrochloric acid production. Early studies showed that parietal cell autoantibodies predominantly react with a 60-90 kDa gastric autoantigen, identified as the highly glycosylated beta subunit of the gastric H+/K+ ATPase (EC 3.6.1.3) (proton pump) whereas other studies demonstrated that these autoantibodies primarily target the 95 kDa alpha subunit of the pump. Currently the reactivity of parietal cell autoantibodies with the two subunits of the gastric H+/K+ ATPase is well established.
Clinical significance
Parietal cell autoantibodies are detected in up to 90% of patients with pernicious anemia, a chronic disease in the final phase of type A chronic atrophic gastritis. Type A atrophic gastritis (autoimmune) is associated with pernicious anemia, whereas type B atrophic gastritis is associated with Helicobacter pylori. However, in Helicobacter pylori-positive patients, the levels of APCA are found to be higher in patients with severe atrophy than in those with mild atrophy [2]. Pernicious anemia usually associates with other tissue-specific autoimmune diseases, like Hashimoto’s thyroiditis, primary Addison’s disease, type I diabetes mellitus and primary biliary cirrhosis. The role of these antibodies in patients with primary biliary cirrhosis seems to be diagnostic rather than pathogenic [3].
Screening methods
Indirect immunofluorescence on human gastric mucosa was the first method used for identifying anti-parietal cell antibodies, back in the early 1960s [4]. Nowadays, these antibodies are routinely detected in clinical laboratories by immunofluorescence on rodent stomach cryostat sections (Figure 2). It is important that the stomach sections maintain the appropriate orientation of the mucosa to properly distinguish the parietal cells from the other cellular types present in the gastric gland.
Sections of rat gastric parietal cells have been pointed out as one of the test substrates showing what is considered one of the classical heterophile immunofluorescent patterns [5]. However, the use of mouse instead of rat stomach sections to prevent the occurrence of false positives due to the presence of heterophile antibodies might not be a good approach since a false smooth muscle staining could then show up. Instead the use of multiple tissues in the test substrate could be the most satisfactory solution.
The fluorescent pattern of anti-parietal cell antibody can be easily confused with the anti-mitochondrial antibody staining pattern. However, there are some specific features that can help to avoid such confusion (Figure 3).
In addition to these specific features of these two patterns, the use of combined tissue sections in the same reaction well can be an aid that allows checking the staining on other structures to clearly differentiate between these two antibody types. In kidney/stomach sections, the anti-mitochondrial antibodies show an intense staining of the cytoplasm in the renal tubules, whereas the anti-parietal cell antibodies do not react with the kidney and the image is completely negative (Figure 4). Furthermore, if the reaction well also contains rat liver, the cytoplasm of hepatocytes shows a strongly positive labelling with anti-mitochondrial antibodies and negative with anti-parietal cell antibodies.
Apart from indirect immunofluorescence, ELISA and linear immunoassays are a suitable method for the detection of APCA in patients with pernicious anemia or type A gastritis, with comparable results with those obtained by indirect immunofluorescence on rodent stomach sections [6]. Several types of these immunoassays can be found, using either native or recombinant H+/K+ ATPase as coating antigen.
References
1. Cruchaud A and Juditz E. An analysis of gastric parietal cell antibodies and thyroid cell antibodies in patients with pernicious anaemia and thyroid disorders. Clin Exp Immunol 1968;3(8):771–781.
2. Ito M, Haruma K., Kaya S, Kamada T, Kim S, Sasaki A, Sumii M, Tanaka S, Yoshihara M, Chayama K. Role of Anti-Parietal Cell Antibody in Helicobacter pylori-associated Atrophic Gastritis: Evaluation in a Country of High Prevalence of Atrophic Gastritis. Scandinavian Journal of Gastroenterology 2002; 37(3): 287-293.
3. Wirth HP, Meyenberger C, Ammann R, Blum HE. Parietal cell antibodies in primary biliary cirrhosis: pathogenetic or diagnostic significance? Schweiz Med Wochenschr. 1994;124(19):816-20.
4. Irvine WJ. Gastric antibodies studied by fluorescence microscopy. Quart J Exp Physiol 1963;48:427-438.
5. Hawkins BR, McDonald BL, Dawkins RL. Characterisation of immunofluorescent heterophile antibodies which may be confused with autoantibodies. J Clin Pathol. 1977; 30(4): 299–307.
6. Sugiu K, Kamada T, Ito M, Kaya S, Tanaka A, Kusunoki H, Hata J, Haruma K. Evaluation of an ELISA for detection of anti-parietal cell antibody. Hepatogastroenterology. 2006;53(67):11-4.
The author
Petraki Munujos, PhD
BioSystems S.A.
Barcelona
Catalonia (Spain)