Anti-PLA2R: the serological biomarker for primary MN
Autoantibodies against phospholipase A2 receptors (PLA2R) are a new, highly specific diagnostic marker for primary membranous nephropathy (MN). Detection of anti-PLA2R using easy-to-perform and inexpensive serological assays can indicate primary MN in patients suffering from nephrotic syndrome and secure a differential diagnosis from secondary MN. Anti-PLA2R analysis is also useful for determining the disease activity, assessing the extent of treatment required and monitoring responses to therapy. Anti-PLA2R antibodies can be determined using innovative indirect immunofluorescence and ELISA test systems.
by Jacqueline Gosink, PhD
Primary membranous nephropathy
Primary MN, also known as primary membranous glomerulonephritis or primary MGN, is a chronic inflammatory autoimmune disease of the blood-filtering structures of the kidneys (glomeruli). It is accompanied by a progressive reduction in renal function. The disease manifests the complex nephrotic syndrome, which is characterized by heavy proteinuria, hypoalbuminemia, hyperlipidemia, edema and lipiduria. Primary MN is one of the leading causes of nephrotic syndrome in adults. As proteinuria increases, so does the long-term risk of kidney failure with major morbidity and mortality, especially from thromboembolic and cardiovascular complications. Around a third of patients progress to end-stage renal disease, a third exhibit persistent proteinuria without progression to renal failure, and the remainder experience spontaneous remission. Primary MN is prevalent in all ethnic groups and in both genders, with men over 40 years old being the most frequently affected.
Diagnostic challenge
The diagnosis of primary MN is demanding, as the disease must be differentiated from other nephropathies, especially from secondary MN, which is triggered by an underlying cause such as a malignant tumour, an infection, drug intoxication or another autoimmune disease such as systemic lupus erythematosus or diabetes mellitus type 1. Of all MN cases, 20-30% are of secondary genesis, while the remaining 70–80% are classified as primary. Primary cases with no detectable anti-PLA2R antibodies are subclassified as idiopathic; it has been postulated that these patients may exhibit antibodies against other, as yet unidentified, target antigens. Reliable differentiation of primary and secondary forms of MN is critical because of different treatment regimes: primary MN is treated with immunosuppressants, while therapy for secondary MN is targeted at the underlying disease.
MN is diagnosed by kidney puncture followed by histological examination or electron microscopy of the tissue to identify the characteristic glomerular immune deposits. To obtain a definite diagnosis of primary MN, secondary causes must be excluded, which involves additional time-consuming and often invasive procedures, for example tumour screening. Moreover, in some patients, MN appears before the secondary cause is even detectable, adding an extra layer of complexity to diagnosis and therapeutic decision-making. Primary MN must also be differentiated from other autoimmune diseases with kidney involvement, for example lupus nephritis, vasculitides associated with antibodies against neutrophil cytoplasm (ANCA) and Goodpasture’s syndrome. The availability of reliable serological tests to support the diagnosis of primary MN has been elusive until recently due to lack of knowledge about the target antigen.
New pathognomonic marker
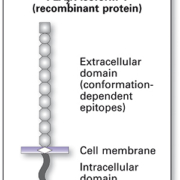
Autoantibodies against PLA2R were first discovered and described in patients with primary MN in 2009 (1). PLA2R is a transmembrane glycoprotein (Figure 1) which is expressed in human glomeruli on the surface of podocytes and is involved in regulatory processes in the cell following phospholipase binding (Figure 2). Type M PLA2R has been identified as the major target antigen of autoantibodies. In patients with primary MN, antigen-antibody complexes form deposits in the glomerular basement membrane, where they trigger local complement activation with overproduction of collagen IV and laminin. This causes damage to the podocytes, via destruction of the cytoskeleton and broadening of the basement membrane. As a result protein enters the primary urine, giving rise to proteinuria and other symptoms.
Differential diagnosis
Autoantibodies of class IgG against PLA2R are present in the serum of up to 70-80% of patients with primary MN (1, 2), whereas they are not found in healthy blood donors or patients with secondary MN or other kidney diseases such as lupus nephritis (3) or IgA nephritis. The high predictive value of anti-PLA2R makes this parameter ideally suited as a diagnostic marker (4).
Disease evaluation
Anti-PLA2R antibodies are, moreover, very sensitive markers of clinical disease activity. They reflect the pathogenic immunological activity of the disease, which is responsible for the clinical expression in the form of proteinuria. High antibody titres indicate a severe disease course (2, 5), while low titres are associated with a decreased risk of renal failure and a greater rate of spontaneous remission (6). Thus, the anti-PLA2R titre also serves as a prognostic indicator.
Therapy monitoring
Treatment with immunosuppressants results in a drop in the anti-PLA2R titre, while in relapse the antibody titre increases again. Significantly, changes in the antibody titre typically precede changes in the proteinuria (6, 7). Thus, a titre increase is detectable before proteinuria appears, while a titre decrease is observed before a reduction in the proteinuria. Patients in remission exhibit residual proteinuria months after the anti-PLA2R titre becomes undetectable. Anti-PLA2R measurements are therefore extremely useful for early therapeutic decision-making and for long-term monitoring of responses to immunotherapy.
A recently published prospective study reinforced the value of anti-PLA2R antibodies as a marker of clinical outcome (8). In the study 133 patients with primary MN were tracked over a time period of 24 months. In all cases there was a clear correlation between proteinuria and anti-PLA2R levels. In patients who were given immunotherapy, a significant time lag was observed between the rapid fall in antibody levels and the protracted reduction in proteinuria. Moreover, remission of proteinuria occurred later in individuals with high antibody levels than in those with low levels. In patients who did not receive immunosuppressive therapy, spontaneous remission was also associated with a reduction in anti-PLA2R, while individuals who did not achieve remission showed continued elevated antibody levels. Thus, anti-PLA2R proved a reliable biomarker for immunological and clinical activity in primary MN.
Risk assessment
Up to 40% of patients with primary MN experience a relapse after kidney transplantation. The risk of recurrent primary MN is particularly high if anti-PLA2R antibodies are found prior to transplantation. In a study on a patient with primary MN, who exhibited high anti-PLA2R levels before and three months after transplantation (7), it could be shown that immunotherapy resulted in a drop in the antibody concentration and also the level of proteinuria. Other studies have shown that anti-PLA2R or PLA2R deposits are detected more often in transplant patients with recurrent MN than in those with de novo MN. A retrospective analysis of fifteen transplant patients revealed that a persistently positive anti-PLA2R activity at six months or later after transplantation was a significant risk factor for relapse, especially in patients on a weak immunosuppressive regimen (9). Thus, the anti-PLA2R antibody titre is useful for assessing the risk of relapse after transplantation and the extent of immunotherapy needed to prevent a recurrence.
Anti-PLA2R test systems
Anti-PLA2R autoantibodies can be determined easily and reliably using standardized indirect immunofluorescence test (IIFT) and ELISA systems. In the IIFT a BIOCHIP of transfected human cells expressing recombinant PLA2R is used as the antigenic substrate to provide monospecific antibody detection (Figure 3). A second BIOCHIP containing cells transfected with an empty vector serves as a control. The IIFT represents an established test for serodiagnostic screening, providing qualitative and semi-quantitative antibody analysis. The corresponding ELISA is based on purified recombinant PLA2R and shows the same high-quality characteristics as the IIFT. The ELISA is particularly useful for disease and therapy monitoring as it offers precise quantification of antibody levels in patient sera. The IIFT and ELISA are fast and simple to perform and are suitable for use in any diagnostic laboratory. Both procedures can be automated.
Clinical data
The performance characteristics of the Anti-PLA2R IIFT and ELISA have been assessed in a multitude of studies. In a retrospective clinical study (10) the Anti-PLA2R IIFT yielded a prevalence of 52% in a cohort of 100 patients with biopsy-proven primary MN, and a specificity of 100% with respect to healthy controls and patients with secondary MN or non-membranous glomerular injury. In a prospective clinical study (11) the sensitivity amounted to 82% in patients with biopsy-proven MN where no secondary cause could be found. The difference in sensitivities obtained in different study panels is most likely due to factors such as disease remission and the therapy status of the individuals, which can influence the antibody results, especially when studies are performed retrospectively.
Results obtained with the ELISA show a very good correlation with results from the IIFT (Figure 4). In a retrospective study with sera from 198 patients with primary MN and 836 healthy and disease controls, the ELISA showed a sensitivity of 96% with respect to the IIFT, and a specificity of 99.9% with borderline sera included (12). The few discrepant sera that were negative in the ELISA gave only low titres of 1:10 to 1:100 in the IIFT. All sera with titres of over 1:100 in IIFT were also positive in ELISA.
Summary
Antibodies against PLA2R represent a landmark development in nephrological diagnostics. Their detection can secure a diagnosis of primary MN in patients with nephrotic syndrome, offering a convenient, non-invasive alternative to biopsy. Anti- PLA2R determination, moreover, yields information about the disease status and evolvement. Serial measurements are especially useful for monitoring therapy responses over the long term and guiding decision-making on the extent of treatment required for individual patients. Serological tests based on state-of-the-art IIFT and ELISA technology provide simple, quick and highly specific anti-PLA2R antibody detection.
References
1. Beck et al. N. Engl. J. Med. 2009: 361: 11-21
2. Hofstra et al. Clin. J. Am. Soc. Nephrol. 2011: 6: 1286-91
3. Gunnarsson et al. Am. J. Kid. Dis. 2012: 59 (4): 585-6
4. Schlumberger et al. Autoimmunity Reviews 2014: 13: 108-13
5. Kanigicherla et al. Kidney Int. 2013: 83: 940-948
6. Hofstra et al. J. Am. Soc. Nephrol. 2012: 23 (10): 1735-43
7. Stahl et al. N. Engl. J. Med. 2010: 363: 496-8
8. Hoxha et al. J. Am. Soc. Nephrol. 2014: 25: 1137-9
9. Seitz-Polski et al. Nephrol. Dial. Transplant. 2014: under revision
10. Hoxha et al. Nephrol. Dial. Transplant. 2011: 26 (8): 2526-32
11. Hoxha et al. Kidney Int. 2012: 82: 797-804
12. Daehnrich et al. Clin. Chem. Acta. 2013: 421C: 213-8
The author
Jacqueline Gosink PhD
EUROIMMUN AG
Seekamp 31
23560 Luebeck
Germany
E-Mail: j.gosink@euroimmun.de