Anti-TNF-α levels and Anti-TNF-α antibodies in inflammatory bowel disease
The introduction of infliximab and adalimumab, monoclonal antibodies against TNF-α, to induce and maintain clinical remission in patients with moderate to severe inflammatory bowel disease has generated new perspectives managing these disorders [1]. However, about a third of all CED patients in clinical studies treated with TNF-α antibodies exhibited no primary response (primary treatment failures) and up to 40% of patients, after having exhibited primary therapy response, show a decrease in efficacy with increasing duration of therapy (secondary treatment failures*) and do need multiple dose adjustments to re-induce or maintain clinical response.
by Dr J. Stein
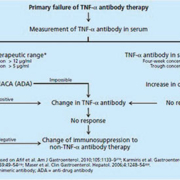
Clinically important factors predicting treatment response include brief disease duration, a predominantly inflammatory disease course and disease involving the colon, non-smoker status and moderate to severe disease activity (overview in Yanai and Hanauer [2]). At first, the development of antibodies against infliximab in the sense of anti-drug antibodies (ADA) [3] occurring especially in patients undergoing episodic administration of IFX (36–61% of cases) [4] was proposed as the primary cause of this phenomenon. It is now considered increasingly questionable that ADA alone are responsible for therapy failure in patients treated with IFX. In fact, multiple studies have reported IFX trough levels that were either undetectable or very low despite the absence of ADA [5-7], which points to other factors that may influence the pharmacokinetics of these agents. A retrospective analysis of the ACT1 and ACT2 studies found an inverse correlation with serum albumin concentrations [8]. Albumin concentrations < 3 g/dl correlated with a significantly poorer initial response (primary non-responders). Several strategies can be undertaken in cases of loss of response: dose escalation (increasing the dose or shortening the interval), switching to another anti-TNF-α drug, or changing to other immunosuppressive drugs. The decision as to which is the best option for the management of these patients remains largely empirical. Data from studies suggests that measurement of anti-TNF-α trough levels and ADAs could be useful in therapeutic drug monitoring in IBD patients, as part of an individualized therapy. Figures 1a and 1b summarize an algorithm for the management of TNF-α antibody therapy based on currently available data. Methods used to detect anti-TNF-α drug concentrations and ADA concentrations are mainly based on enzyme-linked immunosorbent assay (ELISA) and radioimmunoassay (RIA) or less frequently EMSA (electrophoretic mobility shift assay). Compared to the more complex RIA- or EMSA- based detection methods, the most commonly used and, as a rule, easily performed enzyme-coupled immunoabsorptive assays (EIA) exhibit some limitations which are important for the timing of measurement: Since anti-TNF-α drugs are able to bind to antibodies to form immune complexes, they cannot be detected by ELISA and their presence can only be ascertained by detectable ADAs regardless of the serum levels of the anti-TNF-a drug. However, when ADAs are negative, it is important to know the levels of the anti-TNF-α drugs: if anti-TNF-α serum levels are undetectable, the result is a true negative, but if anti-TNF-α levels are detectable and ADA levels are negative, the result is considered inconclusive because it might be either a true negative result or a false negative result if the antibodies have bound to the anti-TNF-α drug. Therefore, anti-TNF-α drug concentrations should be determined when the drug levels are expected to be lowest, i.e. just before the next administration of the drug (trough level) and at the same time antibody titres are measured to enable further interpretation. When an EIA is used, the optimum trough level stands at > 4–5 μg/ml [5,8], compared with cut-off values of > 1 μg/ml with RIA [9,10].
* Defined as recurrence following initially effective remission maintenance with TNF-α antibodies.
References
1. Chaparro M, et al. Aliment Pharmacol Ther 2012; 35: 971–986.
2. Yanai H, et al. Am J Gastroenterol. 2011; 106: 685–98.
3. Baert F, et al. N Engl J Med. 2003; 348(7): 601–608.
4. Cassinotti A, et al. Pract Gastroenterol. 2010; 34:11–20.
5. Maser EA, et al. Clin Gastroenterol Hepatol. 2006; 4:1248–1254.
6. St Clair EW, et al. Arthritis Rheum. 2002; 46: 1451–9.
7. Fasanmade AA, et al. Int J Clin Pharmacol Ther. 2010; 48: 297–308.
8. Seow CH, et al. Gut. 2010; 59: 49–54.
9. Steenholdt C, et al. Scand J Gastroenterol. 2011; 46: 310–318.
10. Bendtzen K, et al. Scand J Gastroenterol. 2009; 44: 774–781.
The author
J. Stein, MD, PhD
Crohn Colitis Centre
Frankfurt, Germany