Autoantibody diagnostics in glomerulonephritis
The determination of autoantibodies is an important component in the diagnosis and differentiation of glomerular disease. Key analyses include antibodies against phospholipase A2 receptors (anti-PLA2R), the glomerular basement membrane (anti-GBM), neutrophil granulocyte cytoplasm (ANCA), double-stranded DNA (anti-dsDNA) and nucleosomes (ANuA). With these tests autoimmune reactions can be identified as causative factors of renal disease.
by Dr Jacqueline Gosink
Glomerulonephritis (GN) is an inflammation of the blood-filtering structures of the kidneys (glomeruli) which can lead to kidney failure if left untreated. The disease is associated with the symptom complexes nephritic syndrome and nephrotic syndrome. Nephritic syndrome is characterised by hematuria, mild to moderate proteinuria and hypertension and is observedain diseases such as post-infectious GN, lupus nephritis, rapid progressive GN and IgA nephropathy. Nephrotic syndrome combines heavy proteinuria, hypoalbuminemia, hyperlipidemia and edema and is typical of membranous GN, minimal change GN and focal segmental glomerulosclerosis.
Because of the wide range of potential causes, the diagnosis of GN can be difficult. The diagnostic process is based on clinical examination, biopsy, and laboratory tests on urine and blood. The serological analysis of specific autoantibodies allows autoimmune forms of GN to be identified and distinguished from nephropathies of other origins, for example hereditary conditions, infections, drug intoxication, electrolyte or acid-base disturbances, diabetes and hypertension.
Autoantibodies in GN may be directed against specific renal targets, such as PLA2R or the GBM, resulting in diseases that predominantly injure the kidneys. Or they may be non-organ-specific, for example ANCA, anti-dsDNA or ANuA. Non-organ-specific autoantibodies cause damage to a wide variety of organs. Thus, GN may represent just one manifestation of a complex systemic autoimmune disease, for example systemic lupus erythematosus (SLE) or ANCA-associated vasculitis (AAV).
Anti-PLA2R antibodies
Autoantibodies against PLA2R are a new and highly specific marker for primary membranous glomerulonephritis (MGN), also known as idiopathic membranous nephropathy. Primary MGN is a chronic inflammatory autoimmune disease of the glomeruli and is one of the leading causes of nephrotic syndrome in adults. It is distinguished from secondary MGN, which is triggered by an underlying disease such as a malignant tumour, an infection, drug intoxication or another autoimmune disease such as SLE. Primary MGN accounts for 70-80% of cases of MGN, while the secondary form comprises around 20-30%. Clinical differentiation of the two forms is crucial since primary MGN is treated with immunosuppressants, whereas therapy for secondary MGN focuses on the causal disease.
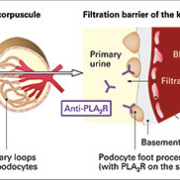
The immune reactions leading to primary MGN, which were first described in 2009 [1], stem from autoantibodies binding to PLA2R (transmembrane glycoproteins, [Figure 1]) on the surface of the podocytes [Figure 2]. PLA2R of type M have been identified as the major target antigen of the autoantibodies. The antigen-antibody complexes are deposited in the GBM, triggering complement activation with overproduction of collagen IV and laminin. This damages the podocytes, resulting in protein entering the primary urine. With increasing proteinuria there is a higher long-term risk of kidney failure with major morbidity and mortality, especially from thromboembolic and cardiovascular complications.
Primary MGN is diagnosed by kidney puncture followed by histological examination or electron microscopy of the tissue to detect immunoglobulin-containing deposits in the GBM. Serological determination of anti-PLA2R antibodies supports the diagnostic procedure and has the advantage of being less time-consuming and less stressful for patients. Anti-PLA2R antibody analysis is, moreover, suitable for monitoring the activity of primary MGN and the response to therapy.
Until recently there was no reliable test to detect anti-PLA2R antibodies. A new recombinant-cell anti-PLA2R indirect immunofluorescence test (IIFT) developed to address this deficit has rapidly established itself as the gold standard for the serological diagnosis of primary MGN. The assay utilizes transfected human cells expressing recombinant PLA2R as the antigenic substrate [Figure 3] to provide monospecific antibody detection [2, 3]. The sensitivity of the test for primary MGN amounts to around 50-80% depending on the characteristics of cohort individuals, for example their disease activity or therapy status. In a retrospective clinical study [2] the Anti-PLA2R IIFT demonstrated a sensitivity of 52% in a cohort of 100 patients with biopsy-proven primary MGN and a specificity of 100% with respect to control subjects. In the first prospective study [4] the sensitivity amounted to 82% in patients with biopsy-proven MGN where no secondary cause could be found. An ELISA based on purified recombinant PLA2R has also been developed. It demonstrates >98% correlation with the IIFT and is particularly useful for quantification of antibody levels in therapy monitoring.
Anti-GBM antibodies
Autoantibodies against GBM are a highly specific and sensitive marker for Goodpasture’s syndrome, a rare, but potentially fatal autoimmune disease which is characterized by rapidly progressive GN and lung haemosiderosis. Diagnosis of this disease is challenging because of the speed of progression to organ failure and the initially unspecific symptoms. Serological parameters such as anti-GBM play a crucial role in obtaining an early diagnosis.
The primary target antigen of anti-GBM antibodies is the NC1 domain of the alpha chain of type IV collagen. The antibodies target the alveolar basement membrane or the GBM. In cases without lung involvement they are detected in more than 60% of patients and in cases with lung involvement in over 90%. Clinical progression of the disease correlates with antibody concentration, with high-titre circulating anti-GBM antibodies indicating an unfavourable prognosis.
Anti-GBM antibodies can be detected serologically by IIFT using sections of primate kidney as the antigenic substrate. Inclusion of a second substrate comprising microdots of purified GBM allows results to be confirmed at a glance. The substrates are positioned side by side as BIOCHIP Mosaics in the test fields of a microscope slide [Figure 4] and incubated in parallel. Further substrates for differential diagnostics, for example HEp-2 cells, granulocytes or other microdot substrates, can also be included in the BIOCHIP Mosaics, yielding a detailed patient antibody profile following a single incubation. Serum anti-GBM antibodies can alternatively be detected or confirmed quantitatively using the Anti-GBM ELISA.
ANCA
ANCA determination is a well-established tool for serological diagnosis and differentiation of different types of AAV, which often present as a rapidly progressive GN among other symptoms. The most important ANCA parameters include antibodies against proteinase 3, which are sensitive and specific markers for Wegener’s granulomatosis, and antibodies against myeloperoxidase (MPO), which occur in microscopic polyangiitis and other forms of AAV.
The standard method for detecting ANCA is IIFT using granulocytes to identify the typical staining patterns of anti-PR3 antibodies (cytoplasmic, cANCA) and anti-MPO antibodies (perinuclear, pANCA). BIOCHIP Mosaics are particularly useful for this application as they allow different substrates to be combined and analysed in parallel [Figure 5]. Recently, several new substrates have been developed to improve the ease and reliability of ANCA analysis still further. HEp-2 cells coated with granulocytes allow immediate differentiation between ANCA and anti-nuclear antibodies, while BIOCHIPs containing microdots of purified MPO or PR3 enable monospecific antibody characterization at the same time as the ANCA screening [5, 6].
Monospecific enzyme immunoassays such as ELISA or immunoblot are used to characterize the specificity of the target antigen. A recent major advance in ANCA ELISA is the development of a novel PR3 diagnostic antigen comprising an optimized mixture of native human (hn) PR3 and designer recombinant PR3 expressed authentically in human cells (hr). An ELISA based on this combined antigen provides unsurpassed sensitivity for the detection of anti-PR3 antibodies – 14% higher than even a capture ELISA (7). The Anti-PR3-hn-hr ELISA thus enhances ANCA diagnostics and is also suitable for long-term evaluation of patients.
Anti-dsDNA and anti-nucleosome antibodies
Anti-dsDNA and ANuA are among the immunological parameters used to diagnose SLE, which counts nephritis among its many and variable manifestations. These two markers provide the highest specificity and sensitivity in the serological diagnosis of SLE.
Anti-dsDNA antibodies are found in 60-90% of patients and represent the most established marker for SLE. A recently developed ELISA provides an exceptionally high sensitivity and specificity for detection of these antibodies owing to the use of a novel coating technology based on highly adhesive nucleosomes. The unspecific reactions that typically occur with traditionally used coating materials are thus avoided, and the clear presentation of the major DNA epitopes ensures a remarkably high sensitivity. In a published clinical comparison study using a large cohort of patients with SLE and other diseases [8], the Anti-dsDNA-NcX ELISA demonstrated the highest sensitivity for SLE (60.8%), exceeding that of conventional ELISA (35.4%), Crithidia luciliae IIFT (27.4%) and even Farr-RIA (53.1%) [Figure 6].
ANuA [Figure 7] are specific for SLE and are a prognostic indicator for SLE with renal involvement. The frequency of ANuA is especially high in severe cases requiring transplantation (79%), compared to less severe lupus nephritis (18%) and SLE without nephritis (9%) [9]. The relevance of ANuA is, however, highly dependent on the assay used to detect them. If insufficiently purified nucleosomes are used in ELISA, then sera from patients with scleroderma or other diseases also frequently react, resulting in an unacceptably low specificity. The 2nd generation Anti-Nucleosome ELISA, in contrast, is based on a patented preparation of highly purified mononucleosomes, which are free of contaminating histone H1, non-histone proteins such as Scl-70, and chromatin DNA fragments. This ELISA provides an SLE specificity of close to 100% and a sensitivity of around 54%. Significantly, with this highly specific test ANuA have been shown to be present in 16-18% of SLE sera that are negative for anti-dsDNA antibodies [Table 1] [10, 11]. Thus, the determination of ANuA substantially enriches the serological diagnosis of SLE. When both ANuA and anti-dsDNA antibodies are analysed in parallel as first-line serological tests, the detection rate for SLE can be increased to 87%.
Conclusions
Recent developments in autoantibody diagnostics for nephrology include the groundbreaking anti-PLA2R IIFT for identifying primary MGN, as well as considerable improvements in the sensitivity, specificity and convenience of tests for ANCA, anti-GBM, anti-dsDNA and ANuA. These advances have boosted the ease, reliability and relevance of autoantibody testing, aiding the diagnosis of autoimmune forms of GN, especially in their early stages. This is crucial to allow the implementation of interventional therapy and prevent the nephropathy progressing to a fatal end stage.
References
1. Beck et al. N. Engl. J. Med. 2009: 361: 11.21
2. Hoxha et al. Nephrology Diagnosis Transplantation 2011: 26 (8): 2526-32.
3. Debiec et al. Nat. Rev. Nephrol. 2011: 7(9): 496-8
4. Hoxha et al. Kidney International. 2012: 82: 797-804
5. Buschtez et al. Zeitschrift für Rheumatologie 2007: Band 66: 43, 10942-10.
6. Damoiseaux et al. JIM 2009: 348: 67-73
7. Damoiseaux J. et al. Ann. Rheum. Dis. 2009; 68: 228-233.
8. Biesen et al. Lupus 2008; 17(5): 506-507.
9. Stinton et al. Lupus 2007; 15: 394-400.
10. Suer et al. J. Autoimmunity 2004: 22: 325-334.
11. Schluter et al. J. Lab Med. 2002; 26: 516-517.
The author
Jacqueline Gosink, PhD
Euroimmun AG
Luebeck, Germany