Ber-EP4 (CD326) testing by flow cytometry: a rationalized algorithm-based approach
Flow cytometry has traditionally been used to identify hemato-lymphoid neoplasms. However, the flow cytometry laboratories that deal with tissues would often receive samples that have an epithelial neoplasm. In our laboratory, we use flow cytometry to identify cells with epithelial differentiation using Ber-EP4 antibody that targets CD326 (EpCAM). We have formulated an algorithm-based approach for the application of this marker. This approach has been elaborated in this article.
by Dr Pranav Dorwal and Dr Helen Moore
Introduction
The use of flow cytometry in the laboratory has traditionally been applied for diagnosing lymphomas and leukemias. The biggest advantage that flow cytometry has over histopathology is a much quicker turn-around-time, as most of the samples are fresh and can be processed right away, unlike a histopathology sample which needs to undergo fixation and processing before it is ready to be examined. Any additional testing on flow cytometry samples can be performed instantly, whereas the same usually requires another day in the histopathology lab. Many of the lymph node malignancies (primary lymphomas versus metastatic involvement) can appear undifferentiated. In these cases, the histopathologist needs the help of a plethora of immunohistochemical markers to reach a diagnosis. The ability to identify samples where non-hematological malignancies are present can be helpful for the treating physician as well as the reporting histopathologist, who can then test with a more dedicated panel. The ultimate aim of this testing is to get an early diagnosis so that patient’s treatment is not delayed.
A large number of markers have been used to identify epithelial differentiation in tumours by immunohistochemistry (IHC), including cytokeratin (CK), carcinoembryonic antigen (CEA), cancer antigen 125 (CA-125) as well as epitopes recognized by the antibodies LeuM1 (anti-CD15 antibody), and MOC-31 and Ber-EP4 antibodies, both of which recognize epitopes on EpCAM (the epithelial cell adhesion molecule). However, most of these are not available for use by flow cytometry. EpCAM (also known as CD326) was first discovered in 1979 and at that time thought to be specific for colonic carcinoma [1]. Ber-EP4 is, therefore, an anti-CD326 antibody which binds to a cell membrane glycoprotein on human epithelia. There is a comprehensive list of tumours that are Ber-EP4 positive, as described by Went et al. and Spizzo et al. [2, 3]. The traditional use of Ber-EP4 in histopathology has been limited essentially for differentiation between adenocarcinoma and malignant mesothelioma [4]. This could be due to the fact that other epithelial markers (such as CK) are expressed more often than the CD326 (EpCAM) in epithelial malignancies and thus are more helpful in lineage determination.
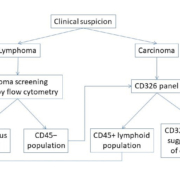
We use an algorithmic approach to decide the flow cytometry panel to be applied (Fig. 1). When the clinical details or radiological findings are indicative of a non-hematopoietic malignancy, we apply the CD326 panel. This panel is composed of CD326, CD56 and CD45. CD56 was included in the panel to identify myeloma cells (which may be present in the CD45-negative region) and cells with neuroendocrine differentiation. If, on analysis, there is no CD45-negative population and the sample is composed of predominantly lymphoid cells, a lymphoid screening panel is then used. Samples that are received with diagnosis of suspected lymphoma are initially processed with a routine lymphoid screening panel. In these cases, Ber-EP4 antibody is tested only if large numbers of CD45-negative events are identified.
Method for Ber-EP4 testing
The tissue and fine-needle aspirate (FNA) samples are received fresh in RPMI medium. The tissues are placed on the metal sieve and ground using a glass pestle to form a cell suspension using 2 % PBS-FCS. This suspension is subsequently filtered, which is then washed and lysed. The cell count is ascertained by the cell counter only in cases of larger tissues, where we may have to dilute the sample to adjust the cell count to approximately 10×109/L. FNA and core biopsies are usually paucicellular and do not need a cell count.
The sample is stained with 5 µl of CD45-PC5 [Immunotech SAS (Beckman Coulter)], 20 µl of CD56-PE (Immunotech SAS) and 10 µl of monoclonal mouse anti-human epithelial antigen-FITC conjugated antibody (Clone: Ber-EP4) (Dako Denmark A/S). The sample is then incubated at 4 °C for 30 minutes, followed by a washing step and is ready to be run on the flow cytometer (Beckman Coulter Life Sciences). A total of 10 000 events are acquired with the time threshold set at 300 seconds for the acquisition.
Flow cytometric analysis
The flow cytometric analysis is performed using Navios and Kaluza softwares (Beckman Coulter Life Sciences). The various populations of interest are gated with the focus on identifying the expression of CD326 (with or without CD56) in the CD45-negative population.
Discussion
In our experience of testing for CD326 by flow cytometry, we have been able to comment on the presence or absence of CD326 expression in CD45-negative populations (Figs 2(a, b) and 3). The various carcinomas where we have identified CD326 positivity are: adenocarcinoma, small cell carcinoma, Merkel cell carcinoma, renal cell carcinoma, squamous cell carcinoma, prostate carcinoma, germ cell tumour of testis, and myxoma. We have observed that the expression of CD326 in melanomas can be variable, but they more frequently express CD56. The co-expression of CD326 and CD56 usually indicates a neuroendocrine tumour. Our concordance rate with histopathology using CD326 testing was found to be 97.6 %, which we have published previously [5].
CD326 expression has also been reported to be a prognostic marker with poor outcomes in epithelial ovarian and gall bladder carcinomas [6, 7]. Another important role of this testing could be application in decision making for use of monoclonal antibodies for targeted therapy. The first EpCAM targeting antibody, Catumaxomab (trade name Removab, Fresenius Biotech GmbH) received European market approval in EpCAM-positive carcinomas for the treatment of malignant ascites. Another modification that could be useful in diagnosing epithelial malignancies is to apply Ki67 testing using flow cytometry. This could be done in the same tube as CD326, and thus more information could be obtained with the same amount of sample [8].
There has been considerable data describing the use of the Ber-EP4 antibody in malignant effusions [9–11]. The literature mentions that the presence of epithelial cells in the body fluid should raise the suspicion of metastatic epithelial malignancy, as the reactive body fluids may be composed of lymphocytes and reactive mesothelial cells in varying proportions. There have been multiple studies in the past where flow cytometric CD326 testing has been applied for identifying epithelial cells in body fluid effusions. We have found that our results have a very good concordance with histopathology results. This is in keeping with the findings of Davidson et al., although their study looked at the detection of malignant cells in effusions [12].
The disadvantage of using Ber-EP4 for identifying epithelial differentiation is that there are many epithelial malignancies that do not express CD326 (EpCAM). As mentioned earlier, the use of a broader antibody like cytokeratin (pan-CK) may solve this problem. But unfortunately, such an antibody is not currently available for clinical use by flow cytometry, to the best of our knowledge. Meanwhile, Ber-EP4 should give us the answer in most of the cases. Another disadvantage is that CD326 will be negative in cases of neoplasms of mesenchymal origin, such as sarcomas.
Most flow cytometry laboratories across the world will liaise with histopathology departments for the diagnosis of non-Hodgkin lymphomas. The use of Ber-EP4-testing flow cytometry may play an important role even in epithelial malignancies. The antibody used by us is a CE-marked antibody for in vitro diagnostics and, thus, requires a limited verification process. We followed the method recommended by the manufacturer. The rapid turn-around-time of flow cytometry results makes it a useful screening tool. Our experience shows that flow cytometric testing for CD326 (EpCAM) can be a useful method for diagnosing non-lymphoid malignancies that are poorly differentiated. We suggest that this method would be more useful if the protocol for its application is set up in consultation with the histopathology department, along with setting up a channel of bilateral communication. The histopathologist, based on the flow cytometry information provided, can then set up a more directed immunohistochemical panel. We would like to emphasize at this stage that the aim of the flow cytometric CD326 testing is not to formally diagnose carcinomas, but to highlight the presence of epithelial cells which may lead to the diagnosis of carcinoma. Final classification obviously remains the role of the histopathologist.
References
1. Patriarca C, Macchi RM, Marschner AK, Mellstedt H. Epithelial cell adhesion molecule expression (CD326) in cancer: a short review. Cancer Treat Rev 2012; 38(1): 68–75.
2. Went PT, Lugli A, Meier S, Bundi M, Mirlacher M, Sauter G, Dirnhofer S. Frequent EpCam protein expression in human carcinomas. Hum Pathol 2004; 35(1): 122–128.
3. Spizzo G, Fong D, Wurm M, Ensinger C, Obrist P, Hofer C, Mazzoleni G, Gastl G, Went P. EpCAM expression in primary tumour tissues and metastases: an immunohistochemical analysis. J Clin Pathol 2011; 64(5): 415–420.
4. Sheibani K, Shin SS, Kezirian J, Weiss LM. Ber-EP4 antibody as a discriminant in the differential diagnosis of malignant mesothelioma versus adenocarcinoma. Am J Surg Pathol 1991; 15(8): 779–784.
5. Dorwal P, Moore H, Stewart P, Harrison B, Monaghan J. CD326 (EpCAM) testing by flow cytometric BerEP4 antibody is a useful and rapid adjunct to histopathology. Cytometry B Clin Cytom 2017; doi: 10.1002/cyto.b.21543.
6. Spizzo G, Went P, Dirnhofer S, Obrist P, Moch H, Baeuerle PA, Mueller-Holzner E, Marth C, Gastl G, Zeimet AG. Overexpression of epithelial cell adhesion molecule (Ep-CAM) is an independent prognostic marker for reduced survival of patients with epithelial ovarian cancer. Gynecol Oncol 2006; 103(2): 483–488.
7. Varga M, Obrist P, Schneeberger S, Mühlmann G, Felgel-Farnholz C, Fong D, Zitt M, Brunhuber T, Schäfer G, et al. Overexpression of epithelial cell adhesion molecule antigen in gallbladder carcinoma is an independent marker for poor survival. Clin Cancer Res 2004; 10(9): 3131–3136.
8. Sikora J, Dworacki G, Zeromski J. DNA ploidy, S-phase, and Ki-67 antigen expression in the evaluation of cell content of pleural effusions. Lung 1996; 174: 303-313.
9. Pillai V, Cibas ES, Dorfman DM. A simplified flow cytometric immunophenotyping procedure for the diagnosis of effusions caused by epithelial malignancies. A J Clin Pathol 2013; 139(5): 672–681.
10. Krishan A, Ganjei‐Azar P, Hamelik R, Sharma D, Reis I, Nadji M. Flow immunocytochemistry of marker expression in cells from body cavity fluids. Cytometry A 2010; 77(2): 132–143.
11. Risberg B, Davidson B, Dong HP, Nesland JM, Berner A. Flow cytometric immunophenotyping of serous effusions and peritoneal washings: comparison with immunocytochemistry and morphological findings. J Clin Pathol 2000; 53(7): 513–517.
12. Davidson B, Dong HP, Berner A, Christensen J, Nielsen S, Johansen P, Bryne M, Asschenfeldt P, Risberg B. Detection of malignant epithelial cells in effusions using flow cytometric immunophenotyping. Am J Clin Pathol 2002; 118(1): 85–92.
The authors
Pranav Dorwal* MBBS, DCP, DNB; Helen Moore MBChB, FRACP, FRCPA
Waikato Hospital, Pembroke St, Hamilton 3204, New Zealand
*Corresponding author
E-mail: Pranav.dorwal@waikatodhb.health.nz