Biomarkers of vascular calcification in patients with impaired kidney function
Chronic renal failure is a disease with a high and increasing prevalence. Currently about 10% of the population of Europe and North America are affected. The disease is associated with a high morbidity and mortality mainly attributed to cardiovascular diseases. In fact patients with more advanced stages of chronic renal failure have a greater risk of dying due to cardiovascular disease than of renal failure itself. Approximately 50% of these patients die from cardiovascular complications.
by Professor Berthold Hocher
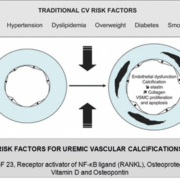
Accelerated vascular calcification (VC) is but one of the important mechanisms of cardiovascular disease in dialysis patients. Under the setting of end-stage renal disease (ESRD), VC is more severe and develops in both the intima and the media of the blood vessels. VC is an active and regulated process mediated by vascular smooth muscle cells [Fig. 1], which undergo a phenotypic change to osteoblasts or chondrocytes, which, in turn, release promoters of VC and apoptosis. VC is markedly up regulated in dialysis patients, and this may be explained by the up-regulation of such promoters of VC as hyperphosphatemia, hypercalcemia, cholesterol, hyperleptinemia down-regulation of the inhibitors of VC such as matrix Gla protein, fetuin-A [1].
Fetuin-A
Fetuin-A is a 62-kilodalton glycoprotein, which belongs to the cystatin superfamily of proteins. In humans, the 349-amino acid protein, as secreted from the liver, consists of two chains: a heavy and a light chain joined by a connecting segment and linked by disulfide bonds. The N-terminus of the heavy chain consists of two cystatin domains, D1 and D2; the acidic amino acids in the D1 domain appear to account for fetuin’s ability to inhibit precipitation of calcium and phosphorus. Indeed, fetuin-A accounts for up to one-half of the in vitro capacity of the serum to prevent the precipitation of calcium and phosphorus. It is now recognised that fetuin-A can actively regulate the cell-mediated process of osteogenesis in the vessel wall, inhibits mineralisation in a concentration-dependent manner, enhances the phagocytosis of apoptotic bodies by vascular smooth muscle cells, limiting their ability to nucleate calcium phosphate. Finally, fetuin-A is an antagonist of bone morphogenetic protein-2, the promoter of VC in vascular cells.
A number of studies have demonstrated an association between serum fetuin-A levels and all-cause mortality of dialysis patients. This association of low fetuin-A levels and mortality was confirmed by clinical trial on 664 hemodialysis (HD) and 323 peritoneal dialysis (PD) patients during a median follow-up of 2.8 years. In this study, an increase in serum fetuin-A by 0.1 g per litre corresponded to a 9% lower death risk. The death predictable value of fetuin-A in this study was independent of serum C-reactive protein (CRP) levels. At the same time, in multivariate analysis of biomarkers of prediction of mortality dialysis patients where serum C-reactive protein was entered, fetuin-A lost its predictable value. The latter fact suggests further investigation of the role of fetuin-A in dialysis patients is needed to fully elucidate the pathomechanisms lowering serum fetuin-A levels in ESRD [1].
Fibroblast growth factor 23 (FGF-23)
FGF-23 is a hormone secreted by osteoblasts. It plays a role in the regulation of phosphorus and in the metabolism of vitamin D. Depletion of FGF-23 causes hyperphosphatemia, up-regulation of 1,25- dihydroxyvitamin D, ectopic calcification and early death. FGF-23 is involved in physiological maintenance of normal serum phosphate levels in the settings of variable dietary phosphorus intake. In the settings of impaired/reduced nephron mass, normal serum phosphate levels are maintained in part by reactive increase of FGF-23, which promotes excretion of phosphate via the remaining nephrons and decreases the absorption of dietary phosphorus by inhibiting the synthesis of 1,25-dihydroxyvitamin D. Depletion of FGF-23 with chronic kidney disease (CKD) progression leads to hyperphosphatemia, ectopic calcification and premature death. It was previously reported that increased serum phosphate levels and decreased 1,25-dihydroxyvitamin D levels are associated with increased mortality.
In the recent study by Gutiérrez et al., multivariable adjusted analyses showed that an increase in serum phosphate levels higher than 5.5 mg/dl and an increase of FGF-23 was associated with a 20% increase in the mortality risk, suggesting hyperphosphatemia and increased FGF-23 are sensitive biomarkers for assessment of the risk of death [reviewed in 1].
Receptor activator of NF-kB ligand–Osteoprotegerin System Osteoblasts regulate differentiation and activation of osteoclasts under conditions of normal bone turnover. Osteoblasts synthesise and secrete a protein called receptor activator of NF-κB ligand (RANKL). RANKL binds to its receptor on pre-osteoclasts and thus regulates bone turnover. Osteoprotegerin (OPG) is also secreted by osteoblasts and modulates the effects of RANKL by blocking osteoblast differentiation. These two key players are also involved in the transformation of vascular smooth muscle cells into bone formatting cells in blood vessels under the condition of chronic renal failure.
Several studies indicate a pathogenic role of OPG in the pathogenesis of cardiovascular diseases in uremic and also non-uremic patients. The OPG/RANKL system plays a key role in the pathogenesis of endothelial function. Tseng et al. suggest that an imbalance between bone formatting hormones and bone degrading hormones may play a key role in the pathogenesis of vascular calcification. High OPG might indicate a reduced degradation capacity of calcified arteries. These authors suggest that an induction of RANKL in the vessel walls might overcome this problem and thus offer even new therapeutic options for vascular calcification. However, this hypothesis needs for sure further investigations [2–4].
Vitamin D
Vitamin D is a multifunctional hormone that can affect many essential biological functions, ranging from immune regulation to mineral ion metabolism. A close association between altered activity of vitamin D and vascular calcification has been reported in various human diseases, including patients with atherosclerosis, osteoporosis and CKD. Experimental studies have shown that excessive vitamin D activities can induce vascular calcification, and such vascular pathology can be reversed by reducing vitamin D activities. The human relevance of these experimental studies is not clear, as vitamin D toxicity is relatively rare in the general population. Contrary to the relationship between vitamin D and vascular calcification, in experimental uremic models low levels of vitamin D were shown to be associated with extensive vascular calcification – a phenomenon that is very similar to the vascular pathology seen in patients with CKD. The current treatment approach of providing vitamin D analogues to patients with CKD often poses a dilemma, as studies linked vitamin D treatment to subsequent vascular calcification. In any case, a close monitoring of the vitamin D status in patients with CKD is indicated to ensure that these patients have vitamin D levels associated with the best survival likelihood [5, 6].
Osteopontin
Osteopontin (OPN) was initially identified in osteoblasts as a mineralisation-modulatory matrix protein. Recently, OPN has been studied as a multifunctional protein that is up regulated in a variety of acute and chronic inflammatory conditions, such as wound healing, fibrosis, autoimmune disease and atherosclerosis. OPN is highly expressed at sites with atherosclerotic plaques, especially those associated with macrophages and foam cells. In the context of atherosclerosis, OPN is generally regarded as a pro-inflammatory and pro-atherogenic molecule. The role of OPN in VC, which is closely related to chronic and active inflammation, is that of a negative regulator. It is an inhibitor of calcification and an active inducer of decalcification. OPN expression and its regulatory molecular mechanisms remain elusive during the process
of vascular calcification. Therefore, further research with regard to the role of OPN in diseases associated with VC is needed to identify potential OPN-related therapeutic targets [7].
References
1. Chaykovska L, Tsuprrykov O, Hocher B. Biomarkers for the prediction of mortality and morbidity in patients with renal replacement therapy. Clin Lab 2011; 57(7–8): 455–467.
2. Shin JY, Shin YG, Chung CH. Elevated serum osteoprotegerin levels are associated with vascular endothelial dysfunction in type 2 diabetes. Diabetes Care 2006; 29(7): 1664–1666.
3. Tseng W, Graham LS, Geng Y, Reddy A, Lu J, Effros RB, et al. PKA-induced receptor activator of NF-kappaB ligand (RANKL) expression in vascular cells mediates osteoclastogenesis but not matrix calcification. J Biol Chem 2010; 285(39): 29925–29931.
4. Ozkok A, Caliskan Y, Sakaci T, Erten G, Karahan G, Ozel A, et al. Osteoprotegerin/RANKL axis and progression of coronary artery calcification in hemodialysis patients. Clin J Am Soc Nephrol 2012; 7(6): 965–973.
5. Lieb W, Gona P, Larson MG, Massaro JM, Lipinska I, Keaney JF Jr, et al. Biomarkers of the osteoprotegerin pathway: clinical correlates, subclinical disease, incident cardiovascular disease, and mortality. Arterioscler Thromb Vasc Biol 2010; 30(9): 1849–1854.
6. Ellam TJ, Chico TJ. Phosphate: the new cholesterol? The role of the phosphate axis in non-uremic vascular disease. Atherosclerosis 2012; 220(2): 310–318.
7. Ketteler M, Rothe H, Krüger T, Biggar PH, Schlieper G. Mechanisms and treatment of extraosseous calcification in chronic kidney disease. Nat Rev Nephrol 2011; 7(9): 509–516.
The author
Berthold Hocher, M.D., Ph.D.
Institute of Nutritional Science, University of Potsdam,
D-14558 Nuthetal-Potsdam, Germany
E-mail: hocher@uni-potsdam.de
www.uni-potsdam.de/eem