Clinical toxicology screening: expanding the capability of highresolution mass spectrometry
High-resolution mass spectrometry (MS) is a powerful analytical tool for clinical and forensic toxicology. It is capable of screening and confirming the presence of drugs and toxic compounds in various biological matrices. Using high-resolution MS/MS libraries helps to increase reporting confidence, verifies compound identification and reduces reporting of false-positive results.
Background
Clinical and forensic toxicology is a highly complex and challenging application domain demanding technologies that can detect a range of illicit drugs, adulterants and therapeutic medications that require screening and identification for medical management. The challenge is further aggravated by the need to accurately identify a compound and negate the reporting of false-positive and false-negative results. Liquid chromatography–tandem mass spectrometry (LC-MS/MS) has been applied successfully to specific, targeted methods in routine therapeutic drug monitoring, such as immuno-suppressants, vitamin analysis, steroids, biogenic amines, psychoactive drugs and synthetic cannabinoids using multiple reaction monitoring (MRM) with triple-quadrupole mass spectrometers. These systems are robust detection technologies and generate high sensitivity and specificity for diverse target panels.
The challenge is to expand target panels particularly with novel psychoactive substances and synthetic illicit substances and to develop methods that can be used routinely for both targeted and non-targeted screening. To meet this need, high-resolution MS is increasingly being applied to routine clinical and forensic toxicology analysis for quanti-fication (using full scan or highresolution MRM) and identification purposes (library confirmation with MS/MS mass fragment spectrum with data-dependent or data-independent acquisitions) in a single analysis.
Application to clinical and forensic toxicology for drugs of abuse in biological samples
Sample preparation
Plasma, serum or blood samples are extracted with a QuEChERS (quick, easy, cheap, effective, rugged, and safe) method [1]. Where possible deuterated internal standards should be included.
LC separation
A validated biphenyl-based separation was developed to ensure that both isobaric targets are chromatographically resolved and the method can be used for a diverse chemical toxicology space with a cycle time of 17 minutes.
MS detection
The method acquired a single time-of-flight (TOF) MS scan (m/z 100−1000) followed by DIA-MS/MS mass scans (m/z 40–1000) in positive ion; each MS/MS mass scan had a precursor isolation width of 20 Da and a collision energy spread of 5−55 V, resulting in a cycle time of approximately 1 second (QTOF LCMS-9030, Shimadzu).
Reporting results with high-resolution LC-MS/MS
Compounds can be identified (LabSolutions Insight software, Shimadzu) with high reporting confidence by combining accurate mass, isotopic distribution, retention time (Rt) and accurate mass fragment spectrum data. A compound can be reported with high
confidence if:
- the target has been detected within a mass error tolerance (typically ± 5 ppm);
- an isotope distribution has a close match with the theoretical distribution;
- an Rt is within a set user tolerance (for example 0.2 minutes); and
- a high similarity score with a curated MS/MS mass fragment spectrum library is found.
The advantage of using a curated MS/MS mass fragment spectrum library
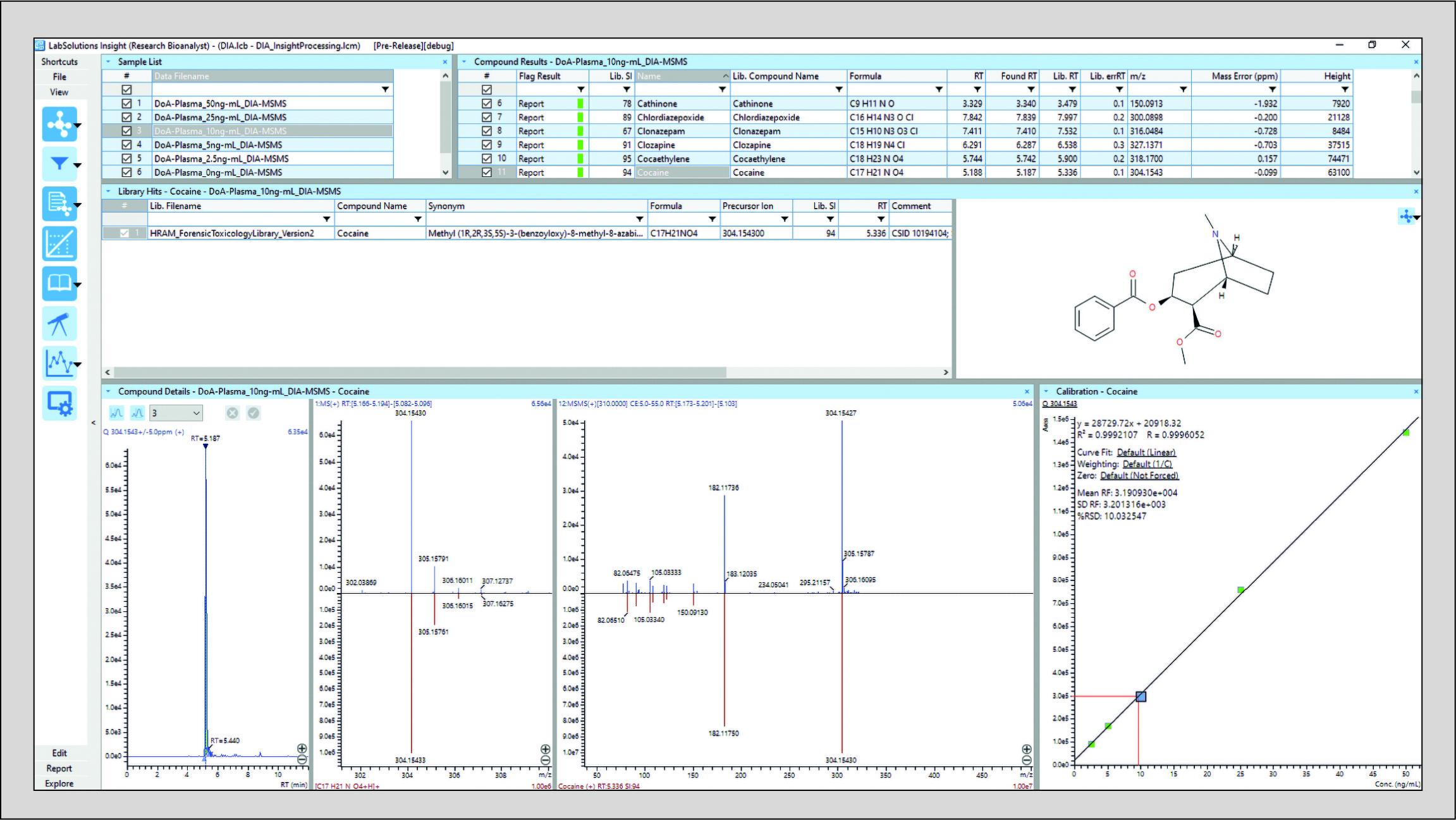
The Insight Forensic Toxicology Database is a repository of over 1000 compounds. Each mass fragment spectrum has been acquired with authentic reference material with known retention times (library data is acquired with a targeted MS/MS and a precursor ion isolation width of 1 Da), and each fragment ion is corrected to a theoretical mass assignment with the Insight Assign application. Using a generic LC method and a single MS and DIA-MS/MS method generates high reporting confidence as a compound identification is confirmed with a limited tolerance on the retention time variation and a high similarity score on the MS/MS library search (Fig. 1). Together with precursor ion accurate mass and isotopic distribution score data, even closely eluting isomers can be reported positively. As the forensic toxicology MS/MS database is matched to the data acquisition in terms of LC retention time and collision energy spread, the library search algorithm similarity score can be a powerful tool to markedly increase reporting confidence and reduce the reporting of false-positive results in routine clinical and forensic toxicology workflows.
The author
Stephane Moreau MSc
Shimadzu Europa GmbH, 47269 Duisburg, Germany
E-mail: shimadzu@shimadzu.eu
References
1. Dulaurent S, El Balkhi S, Poncelet L, Gaulier JM, Marquet P, Saint-Marcoux F.
QuEChERS sample preparation prior to LC-MS/MS determination of opiates,
amphetamines, and cocaine metabolites in whole blood. Anal and Bioanal
Chem 2016; 408(5): 1467–1474.