Colorimetric histology: benefits, opportunities and future directions
Despite rapid advances in the areas of genomics and digital image analysis, cancer diagnosis remains largely reliant on tissue/cellular morphological assessment by an expert pathologist. Here, we summarize a newly developed approach to histology, based on a plasmon-induced colour change which provides instant, label-free visualization (or contrast) of cancer cells, distinguishing them from other cells in a tissue context.
Introduction
The visual inspection of cellular biology under the microscope is an essential step in the diagnosis of a wide range of diseases, including most cancers. In general, the gold standard for pathology assessment is use of nuclear and cytoplasmic stains, typically hematoxylin and eosin (H&E), combined with conventional optical microscopy [1]. Although H&E staining is an excellent tool to visualize the structure of cells, this alone may not provide enough information for an accurate diagnosis for all samples. In particular, when morphology is ambiguous or when there are very small numbers of isolated abnormal cells in the sample, having access to additional biochemical information that can distinguish cellular states could provide critical information to aid in pathology-based diagnosis. This has motivated the use of additional stains specific to gene (fluorescence in situ hybridization, FISH) and protein (immunohistochemistry, IHC) targets that allow additional specificity for disease diagnosis and subtyping. Unfortunately, a marker that reproducibly distinguishes cancer from noncancer is rarely available, and this area is hampered by inter- and intra-patient biological heterogeneity. Here, we describe a biomarkerindependent technology that distinguishes cancer cells in complex tissues based on their physical properties.
How it works
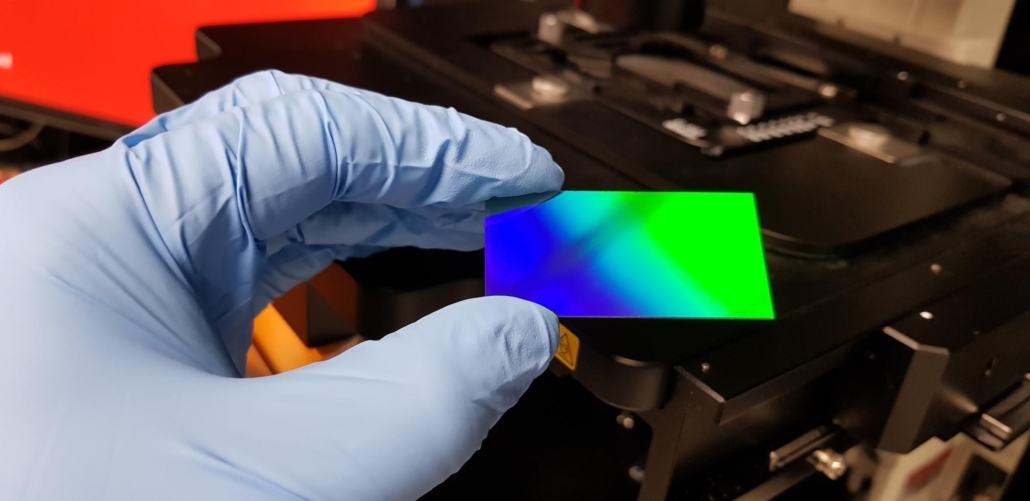
The difficulties in picking up small numbers of cancer cells based on morphology alone, along with the apparent lack of cancer-specific biomarkers, forms part of the underlying motivation for developing a radically different approach to histology: one which offers a high degree of specificity while avoiding the need to perform any chemistry-based staining. Motivated by the desire to improve the accuracy of cancer diagnosis, the authors have spent the past 6 years working on developing a sensor, embedded into the surface of a standard glass microscope slide, which acts a ‘smart’ material that responds to changes in the local cellular composition (Fig. 1).
It is well established in the literature that abnormal (e.g. cancerous) cells interact with light slightly differently to cells which are healthy [2]. The reasons for this are varied and can be specific to a particular sample type; they include differences in the local distribution of protein inside the cell or variations in the stiffness of the cell membrane [3]. The challenge is how to detect these differences, which can often be extremely subtle, without requiring specialist tools or equipment. The solution that we can up with was a ‘smart’ microscope slide which exploits the specific interaction of cells with electrons oscillating within the structure of the sensor to generate characteristic colours (Fig. 2). The colours that are observed under the microscope arise from the fact that tiny changes in cellular composition cause the electrons to oscillate at a different frequency and, thus, emit light at different wavelengths [4]. The slides are designed to work with conventional optical microscopes and are compatible with standard pathology workflows making them easy to use for pathologists without requiring any special tools or training. Rather than relying on the detection of specific antigens that may be produced (for example during abnormal cell proliferation), our ‘colorimetric histology’ technique looks for specific colours which are indicative of certain types of cell. Aside from providing instant biochemical information through induced colorimetric changes in the cell’s appearance, this approach avoids the need for any extended chemical treatment of the sample, which can create a bottleneck in the pathology workflow. It also addresses the lack of effective biomarkers for certain complex disease states (such as some very early-stage cancers) and means that accurate pathology assessments can be made in resource poor locations that may not have access to advanced biochemistry tools.
Current status
Preliminary data obtained using colorimetric histology to analyse a retrospective breast cancer cohort (N=24) spanning non-cancer abnormalities (usual ductal hyperplasia, UDH) and early-stage breast cancer (ductal carcinoma in situ, DCIS) appears extremely promising [5]. Based on pathology scoring using the smart microscope slides, cancer cells could be clearly distinguished from benign, non-cancer states via a clear colorimetric contrast in the epithelial cells. Given that distinguishing these states can be difficult and require additional staining, the ‘smart’ slide holds great promise in early cancer diagnosis. The next steps will be to obtain further validation in a much larger retrospective patient cohort (N>100) and establish whether the implementation of colorimetric histology may help to reduce the requirement for additional stains and the discordance rates in early cancer diagnosis among pathologists. We believe that this technology could have value in many other cancer types, particularly in early diagnosis and where detecting small numbers of cancerous cells may be difficult using morphology alone. The use of colorimetric histology as an adjunct to H&E staining for diagnostic accuracy is also currently being explored. Furthermore applications for colorimetric histology may not be restricted to paraffin-embedded fixed tissues (as used in our proof-of-concept study). Liquid biopsies and even live cells are another area of current research where colour rendition without requiring any fixing and staining offers significant advantages in terms of characterizing cells under near physiological conditions. Finally, intraoperative applications for colorimetric histology are also now being considered where having access to an instant, accurate, diagnosis can have a significant positive impact for patient outcomes.
Interestingly, by exploiting the polarization sensitivity of certain arrangements of plasmonic nanostructures, we have observed that different types of cells can be specifically highlighted while the signal from other cells is suppressed [6,7]. This could further extend the ability of the slides to differentiate specific cells or sub-cellular features in highly heterogeneous samples or enable the pathologist to view certain cells in the colour of their choice. This type of ‘personalized’ diagnostic device could offer a simple solution to further reduce discordance rates which are sometimes dependent on the individual pathologist’s perception of colour.
Challenges and opportunities
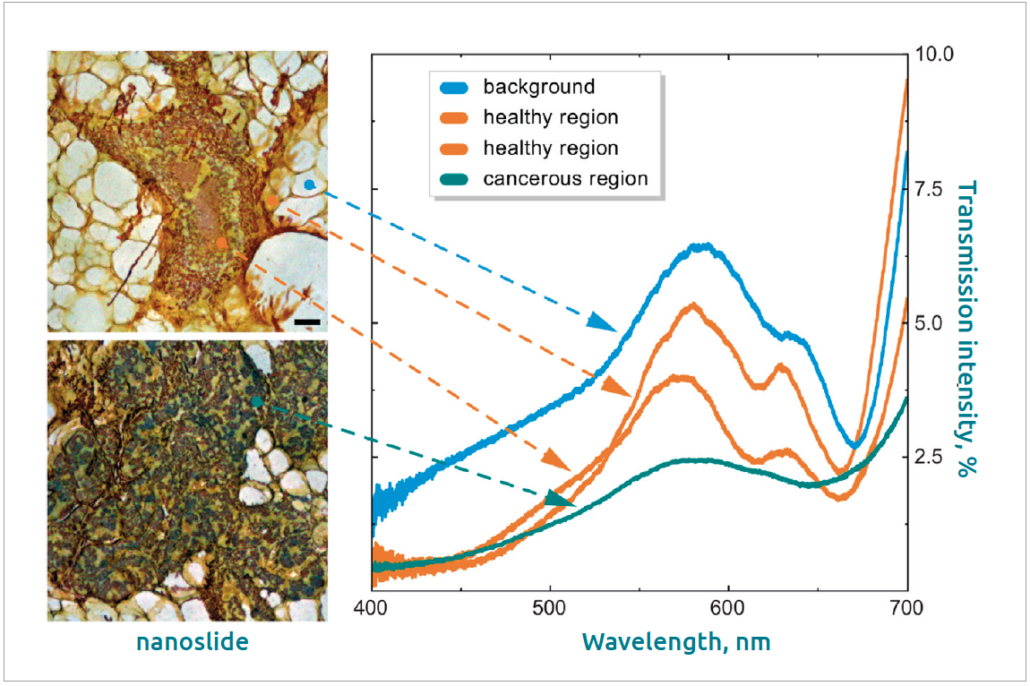
The human eye is incredibly effective at detecting small changes in colour: by exploiting a combination of intensity, colour and morphology, colorimetric histology provides the pathologist with a powerful new set of tools with which to characterize their samples. However, the success of the recent study published in Nature still relied on the expert assessment of a trained pathologist [5]. Although benchmarking against a well-characterized mouse model allowed the authors to build confidence in the colour contrast generated by this technique (Fig. 3), there is still significant work to be done to confirm whether it is possible to achieve an automated diagnosis based on colour alone.
It is likely that if a more accurate approach to digital pathology is developed based on colorimetric histology it will involve the combination of analysis of both morphology and colour. Digital pathology algorithms utilizing whole-slide scanning of H&E-stained slides has already shown great promise as a means of automatically evaluating invasive cancers [8]. However, complex cancers, borderline cases and those of low abundance are a much more significant challenge and in general a trained pathologist with access to both H&E- and IHC-stained slides (where applicable) remains the gold standard for diagnosis. The incorporation of colorimetric histology into digital pathology, however, could address this challenge. The compatibility of the smart microscope slides used for colorimetric histology has already been tested with commercial slide scanners and the slides are archival safe and do not have a ‘shelf life’ meaning that they can either be scanned immediately or subsequently analysed at a later date.
Another opportunity for colorimetric histology lies in its compatibility with other types of imaging techniques and approaches. The method is entirely non-invasive and does not alter the composition or chemistry of the cells in any way. This means that a single tissue can be assessed for biomarker expression or other stains and still be imaged using colorimetric histology, providing a means of obtaining multiple sets of information using the same sample. The ready combination of multiple techniques on a single slide provides the pathologist with the maximum amount of information from which to diagnose and also saves time and reduces the amount of patient tissue required for a definitive diagnosis.
Although there is still much work to be done in order to develop colorimetric histology for in vitro diagnostic medical device regulation (IVDR) in Europe or Food and Drug Administration (FDA) approval for the USA, the high compatibility of the technique with current pathology protocols does offer significant promise of uptake. As of November 2021, preliminary proof-of-concept has been completed in both a small-animal model for early-stage breast cancer and a retrospective DCIS patient cohort. Currently there is no data available for frozen sections, cytology, or non-formalin-fixed paraffin-embedded hematopathology specimens, although these are being pursued as part of the next steps in development. Work is also underway to evaluate pathological concordance using colorimetric histology and compare this to the discordance rate between H&E-based diagnoses and/or associated IHC-stained slides. So far, no consistent inaccuracies due to using colorimetric histology were observed but data from a larger number of patients across multiple sites need to be compared to quantify this. Accurate colour rendition is also essential and protocols for calibration are being developed to account for potential colour deviations between microscopes (e.g. where the source characteristics are varied) using a standardized reference. Such calibration is already embedded in digital pathology protocols which rely on whole-slide imaging using slide scanners for analysis.
Summary
In conclusion, colorimetric histology represents a radically new alternative to conventional histopathology yet is straightforward enough to use that it could form part of the standard pathology toolkit. Switching between H&E-stained slides and our ‘smart’ slides is trivial, enabling confidence to grow in the technology and for pathologists to more easily and quickly reach a definitive diagnosis. Even if colorimetric histology is only used as an adjunct to H&E-stained slides, there are still potentially significant efficiency gains, reduction in labour, and a reduction in the time and costs due to the decreased requirement for ancillary immunohistochemistry that is often ordered after a borderline diagnosis is confirmed. However, although colorimetric histology undoubtedly shows enormous potential as a valuable tool for pathologists, the extent of the clinically relevant benefits needs to be validated in larger-scale studies. In addition, it is difficult to predict at this stage how the technology will be adopted within a hospital and reimbursement setting. Nonetheless, there is currently significant work underway by early adopters and, with further robust, real-world data from much larger-scale patient studies expected in 2022, the advantages and exciting potential of colorimetric histology in the context of pathology is expected to be realized soon.
Figure 1. Photograph of plasmonically active microscope slide used for colorimetric histology Source: B. Abbey, E. Balaur and B. Parker
Figure 2. Comparison of unstained tissue placed on a conventional glass microscope slide compared to being placed on a ‘smart’ microscope slide Source: B. Abbey, E. Balaur and B. Parker
Figure 3. Spectral characteristics of healthy and cancerous tissue taken from the areas shown by the coloured dots Reprinted with permission from Balaur E, O’ Toole S, Spurling AJ, et al. Colorimetric a using plasmonically active microscope slides. Nature 2021; 598(7879): 65–71 [5]. Copyright © 2021 by the Springer Publishing.
The authors
Brian Abbey*1 PhD, Belinda S. Parker2,3 PhD
1Department of Chemistry and Physics, La Trobe Institute for Molecular Science (LIMS), La Trobe University, Melbourne, Victoria, Australia
2Sir Peter MacCallum Department of Oncology, The University of Melbourne, Parkville, Victoria, Australia
3Department of Biochemistry and Genetics, La Trobe Institute
for Molecular Science (LIMS), La Trobe University, Melbourne,
Victoria, Australia
*Corresponding author
E-mail: b.abbey@latrobe.edu.au
References
- Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc 2008; 2008: pdb.prot4986.
- Liu PY, Chin LK, Ser W, et al. Cell refractive index for cell biology and disease diagnosis: past, present and future. Lab Chip 2016; 16(4): 634–44.
- Wang Z, Tangella K, Balla A, Popescu G. Tissue refractive index as marker of disease. J Biomed Opt 2011; 16(11): 116017 (https://bit.ly/3qikbeH).
- Balaur E, Cadenazzi GA, Anthony N, et al. Plasmon-induced enhancement of ptychographic phase microscopy via sub-surface nanoaperture arrays. Nat Photonics 2021; 15(3): 222–229.
- Balaur E, O’ Toole S, Spurling AJ, et al. Colorimetric histology using plasmonically active microscope slides. Nature 2021; 598(7879): 65–71.
- Langley DP, Balaur E, Hwang Y, et al. Optical chemical barcoding based on polarization controlled plasmonic nanopixels. Adv Funct Mater 2018; 28(4): 1704842 (https://bit.ly/3FABM8l).
- Sadatnajafi C, Balaur E, Abbey B. Bimodal plasmonic color filters enable direct optical imaging of ion implantation in thin films. Adv Funct Mater 2021; 2009419 (https://bit.ly/3Fkk4FR).
- Cruz-Roa A, Gilmore H, Basavanhally A, et al. Accurate and reproducible invasive breast cancer detection in whole-slide images: a deep learning approach for quantifying tumor extent. Sci Rep 2017; 7: 46450 (https://go.nature.com/3Jn2HXx).