Developments in cerebrospinal fluid biomarkers for Alzheimer’s disease
Alzheimer’s disease (AD) is the most common cause of dementia. It is characterized by accumulation of β-amyloid (Aβ) peptides and tau proteins, loss of neurons and cognitive decline. It is difficult to diagnose AD in the early stages by clinical examination. Cerebrospinal fluid (CSF) biomarkers can be used to overcome this and could be useful in clinical practice, research and in trials of novel treatments.
by Philip Insel, Dr Rik Ossenkoppele and Dr Niklas Mattsson
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease that leads to cognitive impairment and ultimately dementia. The majority of the 45 million dementia patients world-wide have dementia due to AD [1]. In terms of brain pathology, AD is characterized by progressive accumulation of extracellular deposits of β-amyloid (Aβ) peptides in plaques and intracellular deposits of tau proteins in neurofibrillary tangles. The abnormal metabolism of Aβ and tau is believed to lead to impaired brain function, loss of synapses and neurons, and cognitive decline. The cognitive domain that is most severely impaired in most AD patients is episodic memory, but other domains such as language, visuo-spatial performance, behaviour and executive function may also become affected. In a subset of patients, deficits in non-memory domains are the dominating (early) features, as we will further discuss below.
Stages of Alzheimer’s disease
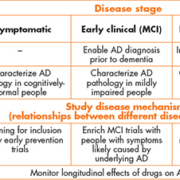
There is an increasing awareness among researchers and clinicians that AD starts many years before the onset of dementia. The first stage is thought to be asymptomatic, when pathologies accumulate silently in the brains of affected individuals for years or even decades before clinical symptoms emerge [2]. This is followed by an early clinical stage, which is characterized by objective cognitive impairment but still preserved function. This is often called mild cognitive impairment (MCI) due to AD, or prodromal AD [3]. The final stage is the classic stage, where AD has caused sufficient functional impairment for the patient to qualify for a dementia diagnosis.
Previously the procedure to diagnose AD was based solely on clinical examination and neuropsychological testing of the patient and interviews with proxies and caregivers. These methods are hampered by low sensitivity in the early stages of the disease and low specificity in the late stages of the disease. With the development of novel imaging and biochemistry technologies it is now possible to diagnose AD with the aid of direct evidence of relevant molecular pathologies in the brain. This is a conceptual leap that has revolutionized clinical AD research and is rapidly transforming clinical practice and clinical trial design. In this article we will discuss this development, with a focus on cerebrospinal fluid biomarkers for AD. Key points are summarized in Table 1.
The first steps
The amino acid sequences of Aβ and tau were identified in the mid-1980s [4, 5]. Early on it was suggested that a test for AD could be developed based on serum measurements of Aβ [4], but it was the discovery in the early 1990s that Aβ is secreted into the CSF that boosted the modern development of biochemical AD markers [6]. In the mid-1990s immunoassays (ELISAs) were developed for CSF Aβ1-42 (the prominent Aβ peptide isoform in Aβ plaques, typically reduced in AD patients compared to controls [7]), total-tau (T-tau, increased in AD patients compared to controls [8, 9]), and phosphorylated-tau (P-tau, increased in AD patients compared to other neurological diseases and controls [8]).
A window into the brain
Traditionally, AD could only be diagnosed at the dementia stage, and definite diagnosis was only possible through post-mortem analysis of brain tissue. The CSF biomarkers Aβ1-42, T-tau and P-tau (and imaging technologies not covered in this article) have made it possible to approach identification of AD brain pathology in living patients. Several studies have found that CSF Aβ1-42 is strongly related to the presence of brain Aβ pathology, quantified at autopsy [10] or in vivo using positron emission tomography imaging with tracers specific for fibrillar Aβ [11]. Likewise, although with less strong associations, CSF P-tau correlates with neocortical tangle pathology [12, 13], whereas CSF T-tau is more non-specifically increased in a number of neurological diseases, with the magnitude of the increase correlating with the size of the damaged tissue and the clinical outcome [14, 15]. CSF biomarkers enable detection of AD pathology in patients in early stages of the disease, when only mild symptoms or no clinical symptoms are present. As clinical diagnosis alone is inadequate in those early disease stages, biomarkers may be critical for an accurate diagnosis. CSF biomarkers may also increase the diagnostic accuracy of the diagnosis in advanced clinical stages, by providing biological evidence of AD related pathology. This helps in identifying the clinical syndrome and differentiating it from other neurological diseases.
Challenges
The field is rapidly advancing to overcome some hurdles that have prevented widespread implementation of CSF biomarkers. Problems with between-laboratory and between-assay variability in measurements have been noticed [16] and are being tackled by the development of certified reference procedures (based on selected reaction monitoring mass spectrometry [17]) and certified reference materials (created by the Institute of Reference Materials and Methods [18]). Development of fully automated assays will further bring down the variability and facilitate implementation of CSF biomarkers outside of expert centres (see www.neurochem.gu.se/TheAlzAssQCprogram for updated comparisons of different assay systems in a global quality control programme). In some countries a remaining obstacle is the unwillingness of medical practitioners to perform lumbar punctures, especially outside of highly specialized clinics. More training is needed to increase the familiarity of doctors with this procedure, and more education is needed to inform staff and patients that the procedure is safe. Headache is the only common complication (2–5 % incidence), but this is usually benign and treatable by common analgesics. Severe complications are extremely rare.
Alzheimer’s disease variants
CSF biomarkers of Aβ and tau may be particularly helpful to assist the diagnostic process in patients with a non-amnestic presentation of AD who may show substantial clinical overlap with patients experiencing non-AD types of dementia. Recently, there has been an increased awareness of these atypical presentations such as posterior cortical atrophy (PCA, ‘visual variant AD’ [19]), logopenic variant primary progressive aphasia (‘language variant AD’ [20]), and the behavioural/dysexecutive variant of AD [21]. As previous studies with small sample sizes have yielded conflicting results, we performed a study in 176 patients selected for abnormal CSF Aβ biomarkers to assess whether CSF T-tau and P-tau differ between atypical variants of AD [22]. Bootstrapping showed that the prevalence of abnormal T-tau and P-tau was ~80–90%, roughly equally distributed across AD phenotypes. This suggests that CSF T-tau and P-tau are equally useful in all clinical phenotypes of AD, which is compatible with current National Institute on Aging–Alzheimer’s Association (NIA-AA) and International Working Group for New Research Criteria for the Diagnosis of AD (IWG-2) diagnostic criteria.
Biomarkers in clinical trials
Biomarker measurement is a recent addition to AD clinical trials. The use of biomarkers is thought to improve trial design both in terms of subject selection and measurement of disease progression. It becomes particularly important in trials of disease-modifying treatments to recruit only those subjects with the target pathology of the therapy. Several recent failed trials may have been hindered by the inclusion of subjects without the underlying pathology [23]. Biomarkers are also less affected by measurement error compared with clinical outcomes and thus offer certain advantages in measuring progression over time, especially in early stages of disease [24]. In these early stages of the disease, before the onset of clinical symptoms, the ability of biomarkers to predict future pathology and cognitive decliners will aid in identifying those most in need of treatment. This will become especially important if intervention in the earliest stages of the disease, prior to substantial neurodegeneration, offers the best chance of a treatment to be effective.
Early treatment trials of anti-Aβ therapies employ thresholds to ensure recruitment of subjects with a minimal level of Aβ pathology. This threshold is frequently taken to be the level of amyloid pathology that most accurately distinguishes cases of AD from cognitively-normal controls [25]. However, if earlier treatment has a higher likelihood of success, identifying subjects with normal amyloid levels who are likely to have elevated levels in the future may be a further step toward early intervention. A recent study demonstrated that amyloid-negative subjects with low levels of CSF Aβ1-42 were much more likely to become amyloid-positive in the near term [26]. Individuals with CSF Aβ1-42 levels in the low normal range may be optimal candidates for early intervention trials aimed at halting further Aβ accumulation.
Conclusions
CSF biomarkers have helped to transform the diagnosis of AD from a clinical diagnosis to a biomarker-informed diagnosis based on molecular evidence of the underlying neuropathology. This has implications for research, where CSF biomarkers enable researchers to characterize subjects at all levels of cognitive function, in clinical practice, where CSF biomarkers aid doctors in diagnosis of AD versus other causes of cognitive impairment, and in the design of clinical trials, where CSF biomarkers may be used to enrich study populations and construct sensitive measures of outcomes to increase study power.
References
1. Alzheimer’s disease International: World Alzheimer Report 2015 (http://www.alz.co.uk/research/world-report-2015).
2. Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis.
JAMA 2015; 313: 1924–1938.
3. Albert MS, DeKosky ST, Dickson D, Dubois B, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7: 270–279.
4. Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984; 120: 885–890.
5. Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, et al. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986; 261: 6084–6089.
6. Seubert P, Vigo-Pelfrey C, Esch F, Lee M, et al. Isolation and quantification of soluble Alzheimer’s beta-peptide from biological fluids. Nature 1992; 359: 325–327.
7. Motter R, Vigo-Pelfrey C, Kholodenko D, Barbour R, et al. Reduction of beta-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer’s disease. Ann Neurol. 1995; 38: 643–648.
8. Blennow K, Wallin A, Agren H, Spenger C, et al. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol. 1995; 26: 231–245.
9. Vandermeeren M, Mercken M, Vanmechelen E, Six J, et al. Detection of tau proteins in normal and Alzheimer’s disease cerebrospinal fluid with a sensitive sandwich enzyme-linked immunosorbent assay. J Neurochem. 1993; 61: 1828–1834.
10. Strozyk D, Blennow K, White LR, Launer LJ. CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003; 60: 652–656.
11. Fagan AM, Mintun MA, Mach RH, Lee SY, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006; 59: 512–519.
12. Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009; 66: 382–389.
13. Seppala TT, Nerg O, Koivisto AM, Rummukainen J, et al. CSF biomarkers for Alzheimer disease correlate with cortical brain biopsy findings. Neurology. 2012; 78: 1568–1575.
14. Hesse C, Rosengren L, Andreasen N, Davidsson P, et al. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci Lett. 2001; 297: 187–190.
15. Otto M, Wiltfang J, Tumani H, Zerr I, et al. Elevated levels of tau-protein in cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. Neurosci Lett. 1997; 225: 210–212.
16. Mattsson N, Andreasson U, Persson S, Carrillo MC, et al. CSF biomarker variability in the Alzheimer’s Association quality control program. Alzheimers Dement J Alzheimers Assoc. 2013; 9: 251–261.
17. Leinenbach A, Pannee J, Dülffer T, Huber A, et al. Mass Spectrometry-Based Candidate Reference Measurement Procedure for Quantification of Amyloid-β in Cerebrospinal Fluid. Clin Chem. 2014; 60: 987–994.
18. Mattsson N, Zetterberg H. What is a certified reference material? Biomark Med. 2012; 6: 369–370.
19. Crutch SJ, Lehmann M, Schott JM, Rabinovici GD, et al. Posterior cortical atrophy. Lancet Neurol. 2012; 11: 170–178.
20. Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, et al. Classification of primary progressive aphasia and its variants. Neurology 2011; 76: 1006–1014.
21. Ossenkoppele R, Pijnenburg YAL, Perry DC, Cohn-Sheehy BI, et al. The behavioural/dysexecutive variant of Alzheimer’s disease: clinical, neuroimaging and pathological features. Brain J Neurol. 2015; 138: 2732–2749.
22. Ossenkoppele R, Mattsson N, Teunissen CE, Barkhof F, et al. Cerebrospinal fluid biomarkers and cerebral atrophy in distinct clinical variants of probable Alzheimer’s disease. Neurobiol Aging 2015; 36: 2340–2347.
23. Karran E, Hardy J. Antiamyloid therapy for Alzheimer’s disease—are we on the right road? N Eng J Med. 2014; 370: 377–378.
24. Hendrix SB. Measuring clinical progression in MCI and pre-MCI populations: Enrichment and optimizing clinical outcomes over time. Alzheimer’s Res Ther. 2012; 4: 24.
25. Shaw LM, Vanderstichele H, Knapik‐Czajka M, Clark CM, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009; 65: 403–413.
26. Mattsson N, Insel PS, Donohue M, Jagust W, et al. Predicting reduction of cerebrospinal fluid β-amyloid 42 in cognitively healthy controls. JAMA Neurol. 2015; 72: 554–560.
The authors
Philip Insel1,2,3 MSs; Rik Ossenkoppele4,5 PhD; Niklas Mattsson*1 MD, PhD
1 Clinical Memory Research Unit, Faculty of Medicine, Lund University, Lund, Sweden
2 Center for Imaging of Neurodegenerative Diseases, Department of Veterans Affairs Medical Center, San Francisco, CA, USA
3 Department of Radiology and Biomedical Imaging, University of California, San Francisco, CA, USA
4 Alzheimer Center and Department of Neurology, Neuroscience Campus Amsterdam, VU University Medical Center, Amsterdam, the Netherlands
5 Memory and Aging Center, University of California San Francisco, San Francisco, CA, USA
*Corresponding author
E-mail: Niklas.mattsson@med.lu.se