Diagnosing diabetes mellitus in Asia using HbA1c
The prevalence of diabetes mellitus in Asia is rapidly increasing. Diabetes in Asia develops in a shorter time, at a younger age and in individuals with a lower body mass index than in the West. This review summarises the epidemiology and pathophysiology of diabetes in Asia as well as the difficulties and challenges in using HbA1c as a diagnostic test in the region.
by Dr R. C. Hawkins
The worldwide rates of diabetes mellitus have more than doubled in the last 30 years, driven by diet, obesity and lifestyle factors [1]. The International Diabetes Federation predicts that the number of individuals affected by diabetes will increase from 240 million in 2007 to 380 million by 2025 [2]. Diabetes is an important health priority in Asia involving huge individual, societal and national costs. Agreement as to appropriate criteria for diagnosis is essential for patient management as well as healthcare planning and epidemiological discussion.
Epidemiology of diabetes in Asia
Asia is a heterogeneous region in terms of ethnicity, culture and socioeconomic development and great differences are seen in the prevalence of diabetes both between and within Asian countries. As the world’s most populous region, Asia is projected to comprise more than 60% of the global diabetic population by 2025. The effects of high population growth, ageing and increasing urbanisation suggest that India and China will remain the two countries with the highest numbers of people with diabetes (79 million and 42 million, respectively) by 2030 [3]. Other Asian countries (Indonesia, Pakistan, Bangladesh, and the Philippines) are in the top ten countries for diabetes prevalence. The prevalence of impaired glucose tolerance in Southeast Asia was estimated as 6.0% in 2007, suggesting that there is a substantial population pool at risk of developing overt diabetes. Differences between ethnic groups may be seen in a single country. In Singapore, a nationwide study in 1998 showed the prevalence of diabetes to be highest in the Indian population (12.8%) followed by Malays (11.3%) and Chinese (8.4%). Age-specific prevalence also varies between Asian populations, with a peak in Indians at 60-69 years compared to 70-89 years in Chinese. South Indians have a higher age-specific prevalence and prevalence of impaired glucose tolerance at a younger age than Chinese. A particular characteristic of diabetes in Asia is the high prevalence of young-onset diabetes. The prevalence of diabetes in China in the 35-44 year age group rose by 88% between 1994 and 2000 while the fraction of diabetics under 44 years of age rose from 25% to 35.7% in South India between 2000 and 2006. Rates of diabetes in children across the region also show alarming rises.
Pathophysiology of diabetes in Asia
The prevalence of insulin resistance and metabolic syndrome is high in Asian people. Although Asian populations have lower rates of obesity than Western populations as defined by the conventional body mass index cut-offs, the prevalence of diabetes in Asians often matches or exceeds that in the West.
Compared to Europeans, Asians have lower muscle mass and increased abdominal obesity and visceral fat. Obesity is associated with many of the metabolic mechanisms that worsen insulin tolerance. Excess lipolysis leads to elevation of blood non-esterified fatty acids and triglycerides, suppressing glucose uptake by muscle. Obesity can also diminish insulin action through leptin and adiponectin secretion. This difference in body fat varies between Asian ethnicities and explains in part the differences in diabetes prevalence seen between ethnic groups. For example, for the same age and BMI, Singaporean Indians have the greatest percentage of body fat, followed by Malays and then Chinese; an order reflecting the different prevalence of diabetes in each group. All three ethnicities have higher body fat percentages than Europeans. The World Health Organisation convened an expert consultation to review the BMI cut offs to define risks in Asian populations and recommended that for Asians, BMI of 23 kg/m2 or higher marks a moderate increase in risk while a BMI of 27.5 kg/m2 or more represents high risk. Several Asian countries have subsequently adopted lower BMI cut-offs than those used in the West. India and Japan use BMI cut-offs of 23 and 25 to define overweight and obese categories while Singapore uses 23 and 27.5 kg/m2.
Genetic differences in risk allele frequencies and location for diabetes genes between Asian and European populations may contribute to some of the physiological differences observed. Asians, especially from Southeast Asia, show higher postprandial glucose concentrations and lower insulin sensitivity than Europeans following a glucose challenge despite controlling for age, BMI, waist circumference, birth weight and diet. Frequency differences between Asian and European populations include transcription factor 7-like 2 gene TCF7L2 (rs7901349) with a minor allele frequency of 0.03 in Asians and 0.27 in Europeans and the potassium voltage-gated channel, subfamily Q, member 1 gene KCNQ1 (rs2237892) with allele frequency of 0.28-0.41 in East Asians and 0.05-0.07 in Europeans. The effect of the same genetic variant may also vary between populations. For example, the effect of the FTO variant is linked to changes in fat mass and obesity in Europeans but its link to body size is weaker in Indian populations.
Nutritional factors may also play a role in the increased rates of diabetes seen in Asia. Increasing urbanisation across Asia has seen an increase in consumption of animal products and fat. Some traditional food sources such as ghee (clarified butter), which is high in trans fatty acids, and polished rice and refined wheat (which have high glycaemic indices) may contribute to obesity and glucose intolerance. Such dietary factors, coupled with an increasingly sedentary lifestyle (e.g. replacement of bicycles with automobiles across Asia) and high cigarette smoking rates (which increases insulin resistance), are helping fuel diabetes rates across the region.
Diagnosis of diabetes mellitus in Asia
Diabetes mellitus has traditionally been defined as a state of chronic hyperglycaemia and measurement of blood glucose concentrations has been the gold standard criterion for diagnosis. The International Expert Committee recently recommended that diabetes should be diagnosed when HbA1c is ≥6.5% and this approach has been adopted by the American Diabetes Association [4].
HbA1c results from the reaction of glucose with the N-terminal valine of the β chain of haemoglobin to form the aldimide (Schiff base or labile HbA1c), which then undergoes an Amadori rearrangement to the stable ketoamine (HbA1c) [5]. To compensate for variation in the total haemoglobin concentration, HbA1c is expressed as a ratio (HbA1c/total haemoglobin). There are a variety of analytical methods for HbA1c determination, including those based on charge differences (e.g. ion-exchange chromatography, electrophoresis, capillary electrophoresis and isoelectric focusing) and structural differences (e.g. affinity chromatography, immunochemical and enzymatic assays). Methods based on charge differences face potential interferences from other haemoglobin moieties (e.g., Schiff base, carbamylated haemoglobin, and haemoglobin variants). Affinity chromatography measures all glycohaemoglobin bound to the resin, not just HbA1c, and thus results are not specific for HbA1c. However as the ratio of HbA1c to all glycohaemoglobin is fixed, it is possible to standardise the reporting to HbA1c equivalent units. Immunoassays are generally less affected by the structural changes of haemoglobin variants than other methods.
There are more than 700 known haemoglobin variants and about half of these variants are clinically silent. The prevalence and type of haemoglobinopathy in Asia differs from that seen in Western populations. Both α and β haemoglobin chain variants can interfere with HbA1c measurement [6]. Common haemoglobin variants seen in Asia affecting HbA1c measurement include HbE, HbC and HbS. Thalassaemia is also endemic in the region and may be accompanied by an increase in HbF concentration. High proportions of HbF compared to HbA can cause falsely low values for HbA1c when the ratio of HbA1c to total haemoglobin is calculated. Red cell lifespan can also be reduced in thalassaemia, causing falsely low HbA1c values. Since many commercial HbA1c methods have been optimised for Western settings, ensuring accurate measurement in Asian populations can be an analytical challenge for the laboratory.
The suggestion that diabetes should be diagnosed when HbA1c is ≥6.5% has led to increasing study in differences in HbA1c between ethnic groups. HbA1c has been shown to be higher in non-Caucasian populations, including Hispanics, Asians and Africans. In a study of Caucasian, Chinese, Malay, Eurasian and Indian outpatients in Singapore, we have shown that HbA1c in all Asian groups was higher than the Caucasian group when corrected for age, sex and fasting glycaemic concentration [7]. Compared to the Caucasian group, Malays and Indians had the highest HbA1c (both 0.6 %HbA1c units higher), followed by Eurasians and Chinese (both 0.3%). The reasons for these ethnic differences in HbA1c are unclear, with potential mechanisms including biological (e.g. glycation and erythrocyte survival) and social (cultural/economic) factors.
We recently examined the performance of HbA1c compared to oral glucose tolerance testing (OGTT) for the diagnosis of diabetes in our outpatient population. Details of OGTT with paired HbA1c samples from 2005-11 were extracted from the laboratory database for statistical analysis. OGTT (75 glucose) was performed and interpreted according to WHO recommendations with 0 and 120 min sampling. All HbA1c (turbidometric immunoinhibition method) and glucose measurements were performed on Beckman Coulter LX20 PRO analysers. Diabetes mellitus was diagnosed using three different methods: (1) fasting plasma glucose ≥ 7.0 mmol/L, (2) 120 minute post prandial plasma glucose ≥ 11.1 mmol/L and (3) fasting plasma glucose ≥ 7.0 mmol/L or 120 minute post prandial plasma glucose ≥ 11.1 mmol/L.
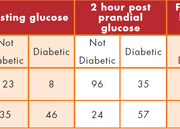
There were 212 records available with average age 57y (29-95); 134 men, 168 Chinese, 28 Indian, 16 Malay. The prevalence of diabetes by (1) was 25%, by (2) was 43%, by (3) was 48% and using a HbA1c cut-off of 6.5% was 38%. The AUC for HbA1c to predict diabetes [see Figure 1] by (1) was 0.89 (95% CI: 0.83-0.94), by (2) was 0.74 (0.67-0.81) and by (3) was 0.78 (0.72-0.85). At the suggested cut-off of HbA1c 6.5% [see Table 1], concordance, kappa and phi values between diabetes diagnosis by HbA1c and (1) was 78%, 0.54, 0.64; (2) 72%, 0.43, 0.44; and (3) 74%, 0.48, 0.50. Maximum efficiency for HbA1c in detecting diabetes as defined by (3) was 0.75 at a cut-off of 6.2% (sensitivity 0.804 (0.714-0.876), specificity 0.691 (0.596-0.776)). There was no significant difference in the age, sex or ethnic makeup of the diabetic groups identified by HbA1c testing or by the other three criteria. Thus there was significant discordance of diabetic classification between use of HbA1c and the OGTT results with a 10% absolute reduction in diabetes prevalence with use of a HbA1c cut-off of 6.5% alone. Thirty-seven percent of OGTT-positive individuals would not be identified by a HbA1c cut-off of 6.5% while 15% of OGTT-negative individuals would be identified as diabetic. These results suggest that the two approaches are not interchangeable and appear to identify different patient groups.
This small study supports reports from large epidemiological studies of lack of agreement between HbA1c- and glucose-based diagnosis and lower prevalence rates with the HbA1c-based criterion [8-10]. Moving to HbA1c-based diagnosis of diabetes can be anticipated to have a substantial effect on prevalence rates and will identify different individuals from the traditional glucose-based criteria. Given that the epidemiology and pathophysiology of diabetes in Asia differs from Western populations, it is perhaps not surprising if diagnostic criteria may need to be customised to local conditions. The need or desirability for different ethnic-based HbA1c cut-offs is unclear and further work is required to examine HbA1c in different ethnic groups within Asia. As HbA1c and glucose-based criteria appear to identify different groups, a clear algorithm outlining the order of testing is required to clarify the role of HbA1c and glucose-based criteria. Unstandardised use of HbA1c and glucose diagnostic testing will complicate epidemiological study of diabetes, both within and between countries. Although a routine combined approach using both HbA1c and glucose measurements may allow the greatest detection of diabetics, it may not be practical or economically feasible for many in Asia. These problems illustrate the difficulty in global standardisation of disease definitions using biological markers and the need to validate approaches in local ethnic populations before introduction.
References
1. Danaei G et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2•7 million participants. Lancet 2011;378:40
2. Chan JC et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009;301:2129-40
3. Ramachandran A et al. Diabetes in Asia. Lancet 2010;375:408-18
4. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327-34
5. Weykamp C et al. A review of the challenge in measuring hemoglobin A1c. J Diabetes Sci Technol 2009;3:439-45
6. Schnedl WJ et al. Hemoglobin variants and determination of glycated hemoglobin (HbA1c). Diabetes Metab Res Rev 2001;17:94-8
7. Hawkins R. Differences in HbA1c between Caucasians, Chinese, Indians, Malays and Eurasians. Clin Chim Acta 2011;412:1167
8. Malkani S et al. Implications of using hemoglobin A1C for diagnosing diabetes mellitus. Am J Med 2011;124:395-401
9. Kim CH et al. Discordance between fasting glucose-based and hemoglobin A1c-based diagnosis of diabetes mellitus in Koreans. Diabetes Res Clin Pract 2011;91:e8-e10
10. Christensen DL et al. Moving to an A1C-based diagnosis of diabetes has a different impact on prevalence in different ethnic groups. Diabetes Care 2010;33:580-2
The author
Dr Robert C Hawkins
Department of Laboratory Medicine
Tan Tock Seng Hospital
Singapore
e-mail: Robert_Hawkins@ttsh.com.sg