Diagnosis of acute hepatic porphyria
The acute porphyrias are a group of rare genetic metabolic disorders of the heme biosynthesis pathway, that result in the toxic accumulation of porphyrin precursors, notably porphobilinogen and delta-aminolevulinic acid. This article provides an overview of the biochemistry and the diagnosis of acute hepatic porphyria, which is crucial to managing this condition appropriately, preventing and treating acute attacks and reducing the long-term consequences of chronic disease.
by Dr A. Sleigh and Prof. Elizabeth L. Frank
Porphyria
Background
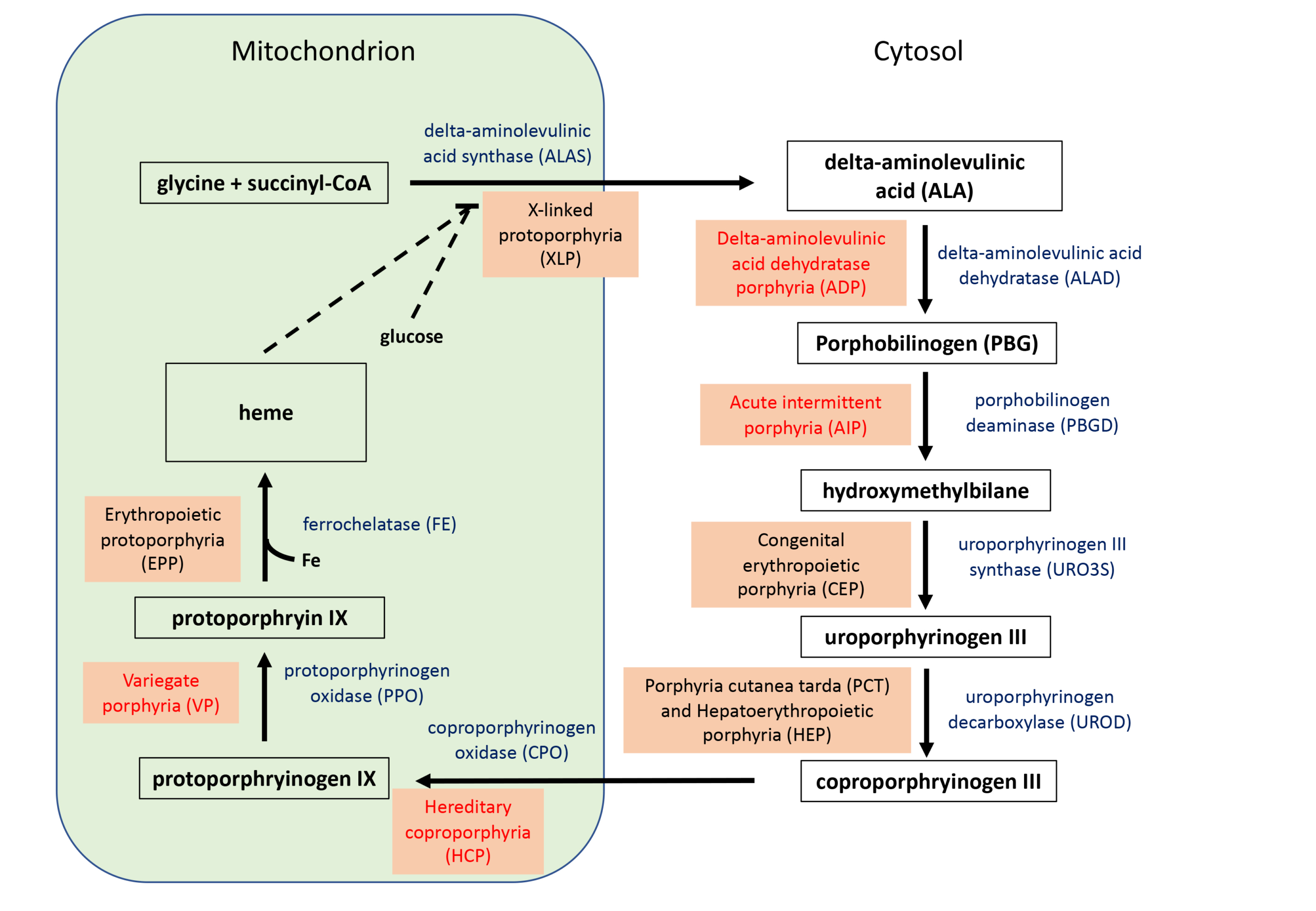
Porphyria is a term that relates to a group of eight rare genetic diseases that cause the accumulation of porphyrins in the body, which result in acute neurovisceral symptoms, skin lesions, or both. Each porphyria is the result of abnormal function (usually deficiency) of the enzymes in the heme biosynthesis pathway (Fig. 1). The acute hepatic porphyrias (AHPs) include acute intermittent porphyria (AIP), variegate porphyria (VP), hereditary coproporphyria (HCP) and the incredibly rare delta-aminolevulinic acid dehydratase deficiency porphyria (ADP). In acute porphyria, the mutant enzyme has enough residual function to allow heme synthesis to occur, but is deficient enough to cause a build-up of the porphyrin precursors. This can be exacerbated by up regulation of delta-aminolevulinic acid synthase (ALAS), which is usually the rate-limiting step in the pathway, by certain therapeutic drugs as well as barbiturates and alcohol.
Incidence
The most common AHP is AIP, and research suggests that the frequency of pathogenic variants in the general population is relatively high, at 1:1600. However, it seems that disease penetrance (the proportion of people with a pathogenic mutation who develop symptoms) is only 0.5–1% [1].
Symptoms and management
In AHP, symptoms are caused by the accumulation of deltaaminolevulinic acid (ALA) and porphobilinogen (PBG). Symptoms can include abdominal pain (reported by 92% of patients), nausea, urine colour change, muscle weakness, tiredness, arm/leg pain, back pain, constipation, vomiting, anxiety and tachycardia (reported by 56% of patients). The majority of patients experience only a few attacks in their lifetime; however, in some patients, symptoms can recur often resulting in impaired life quality, with some experiencing chronic symptoms between attacks.
The long-term complications involve chronic kidney disease, chronic hypertension, hepatocellular carcinoma, polyneuropathy and depression and anxiety. Management involves lifestyle and nutrition modification, treatment of acute attacks with glucose or hemin (triggering negative feedback of ALAS), and treatment of symptoms with appropriate medication for pain, nausea and vomiting. Preventive medication includes gonadotropin-releasing hormone agonists, intravenous hemin, and subcutaneous givosiran, a gene-silencing small interfering RNA (siRNA) directed towards ALAS, which was approved for use by the U.S. Food & Drug Administration in 2019 and has only recently been used in Europe and the UK [2, 3].
Diagnosis
Because AHP is rare and presentation is variable and often intermittent, diagnosis is often delayed or missed. This can result in mismanagement, including the prescription of medications that exacerbate the condition. Laboratory diagnostic testing provides definitive results but only when the correct tests are ordered. Testing standards and guidelines have been developed in recent years in the UK, Europe and Australia, and recommendations have been published in the USA [4–7].
Lab tests
Analytes
Testing a random urine sample for PBG is sufficient to diagnose AHP. Testing for ALA concentrations is no longer needed for diagnosis of AHP, but is useful for discerning tyrosinemia or lead poisoning. In an acute attack, PBG concentrations will be grossly elevated, reaching 10 to 150 times the upper limit of the reference interval and a diagnosis of porphyria is clear [4, 8]. This first-line screening is carried out as the range of symptoms of AHP is so varied and can be caused by other conditions and is often done to rule out AHP. As acute attack of AIP can be life threatening and treatment needs to be started as soon as possible, PBG testing should be carried out on a random (non-timed) urine sample to avoid the delays in diagnosis that can occur with a 24-hour (or total) urine collection. However, if PBG is not elevated but a strong clinical suspicion of porphyria remains, then urine porphyrins should be analysed to prevent a missed diagnosis of VP or HCP, where PBG levels are less elevated and return to normal more quickly [4, 7]. Following a positive first-line test or continued strong suspicion of porphyria, second-line or confirmatory testing should be done to identify the exact type of porphyria. This requires analysis of the original urine sample, EDTA whole blood and feces at (in the UK and Europe) a specialist porphyria lab. Typical results of second-line testing are shown in Table 1. Recommended testing algorithms can be seen in Woolf et al., Anderson et al. and the ARUP Laboratories website [4, 7, 9].
Methods
Quantitative analysis of PBG frequently is performed using ion-exchange chromatography followed by reaction with Ehrlich’s reagent and spectrophotometric detection and was often done using the PBG by Column Test Porphobilinogen kit (Bio-Rad) until its withdrawal from the market. A current alternative is the ClinEasy® Complete Kit for Porphobilinogen in Urine (iris tech/ Recipe). However, rapid and cost-effective in-house tests can be developed using ion-exchange spin columns, as detailed by Roshal et al. [10] and Balogun et al. [11] who also reassessed reference intervals by the analysis of 10 years of de-identified retrospective PBG results.
It is recommended that confirmatory quantitative porphyrin analysis is done in specialist porphyria laboratories that can maintain appropriate staff training and accreditation along with participation in appropriate internal quality control and external quality assessment schemes. This analysis is accomplished using high performance liquid chromatography and fluorescence spectrometry or mass spectrometry techniques [4, 12, 13].
Limitations/caveats/pitfalls
The analytes are light sensitive and therefore need to be protected from light, by collection in amber-coloured tubes or in tubes wrapped in tin foil or black plastic. Samples should be stored at 4°C and are stable for up to four days; feces may also be frozen if sample collection happens before a weekend or bank holiday. Repeated freezethaw cycles should be avoided. If samples need to be sent on to a specialist lab, shipping should happen within 24 hours of collection [4]. As the main specimen type is urine, a waste product that can vary notably in concentration, all quantitative urine results should be reported as a ratio to urine creatinine concentration. Additionally, testing should be carried out on urine samples with creatinine concentrations of at least 2 mmol/L.
What’s next?
Until the 1980s, mortality from acute attacks of porphyria was approximately 25%; however, with early diagnosis and proper treatment, prognosis has much improved. However, a burden of chronic disease in AIP patients is common – particularly chronic liver and kidney disease, and an increased risk of hepatocellular carcinoma in older patients. Now, however, once the porphyria subtype has been identified by biochemical confirmatory testing, the advent of improved gene sequencing technologies allows the identification of the genetic mutation responsible. This can be followed by genetic counselling and mutational analysis of family members, which can identify low levels of disease that might have otherwise been missed or misdiagnosed, and appropriate lifestyle changes/treatment will prevent the accumulation of low levels of chronic disease. Additionally, there is the exciting new development of siRNA therapy givosiran (Alnylam) directed against ALAS, which is now being funded by the NHS in the UK and has revolutionary consequences for the recipients [14].
The authors
Alison Sleigh1 PhD and Elizabeth L. Frank2 PhD, DABCC, FAACC
1Clinical Lab International
2University of Utah Health Sciences Center and Analytic Biochemistry,
Calculi and Manual Chemistry, Mass Spectrometry at ARUP
Laboratories, Salt Lake City, UT, USA
Further resources
1. European Porphyria Network (https://porphyria.eu/).
2. British and Irish Porphyria Network (https://bipnet.org/).
3. ARUP Consult. Porphyrias. ARUP Laboratories
(https://arupconsult.com/content/porphyrias).
References
1. Whatley SD, Badminton MN. Acute Intermittent Porphyria. 2005 Sep 27 [updated 2019 Dec 5]. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH,
et al, editors. GeneReviews® [Internet] (https://www.ncbi.nlm.nih.gov/books/NBK1193/).
2. First treatment for acute hepatic porphyria. European Medicines Agency 2020; 31 January (https://www.ema.europa.eu/en/news/first-treatment-acutehepatic-
porphyria).
3. National Institute for Health and Care Excellence (NICE). Givosiran for treating acute hepatic porphyria [ID1549]. In development [GID-HST10035]Expected
publication date: 24 November 2021 (https://www.nice.org.uk/guidance/indevelopment/gid-hst10035).
4. Woolf J, Marsden JT, Degg T, Whatley S, Reed P, et al. Best practice guidelines on first-line laboratory testing for porphyria. Ann Clin Biochem 2017; 54(2):
188–198 (https://journals.sagepub.com/doi/10.1177/0004563216667965).
5. Stein P, Badminton M, Barth J, Rees D, Stewart MF; British and Irish Porphyria Network. Best practice guidelines on clinical management of acute attacks of
porphyria and their complications. Ann Clin Biochem 2013; 50(Pt 3): 217–223
(https://journals.sagepub.com/doi/10.1177/0004563212474555?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed).
6. Sies CW, Cronin V, Florkowski CM, Gill J, Grant J, et al. Regional variation in analytical techniques used in the diagnosis and monitoring of porphyria: a case for
harmonisation? Clin Biochem Rev 2015; 36(2): 63–74 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4504156/).
7. Anderson KE, Lobo R, Salazar D, Schloetter M, Spitzer G, et al. Biochemical diagnosis of acute hepatic porphyria: updated expert recommendations for primary
care physicians. Am J Med Sci 2021; 362(2): 113–121 (https://www.amjmedsci.org/article/S0002-9629(21)00093-8/fulltext).
8. Bissell DM, Anderson KE, Bonkovsky HL. Porphyria. N Engl J Med. 2017; 377(21): 2017.
9. ARUP Consult. Porphyrias testing algorithm. ARUP Laboratories 2020 (https://arupconsult.com/algorithm/porphyrias-testing-algorithm).
10. Roshal M, Turgeon J, Rainey PM. Rapid quantitative method using spin columns to measure porphobilinogen in urine. Clin Chem 2008; 54(2): 429–431
(https://academic.oup.com/clinchem/article/54/2/429/5628734).
11. Balogun KA, Amundson SA, Weber B, Frank EL. Establishment of a quick method to quantify urinary porphobilinogen and reference intervals using
retrospective laboratory data. Poster presented at AACC 2020.
12. Benton CM, Lim CK, Ritchie HJ, Moniz C, Jones DJ. Ultra high-performance liquid chromatography of porphyrins. Biomed Chromatogr 2012; 26(3): 331–337.
13. Danton M, Lim CK. Porphyrin profiles in blood, urine and faeces by HPLC/electrospray ionization tandem mass spectrometry. Biomed Chromatogr 2006;
20(6-7): 612–621.
14. Gallagher J. Gene silencing medicine transforms crippling pain. BBC News 2021, 21 October (https://www.bbc.co.uk/news/health-58988006).