Diagnosis of celiac disease
Published guidelines provide different approaches for laboratories to follow when investigating celiac disease. The aim is to minimize time to diagnosis and reduce unnecessary investigations. Variability between IgA tissue transglutaminase tests must be considered when implementing the local diagnostic strategy. Determining best practice depends on the assays used, expertise available, cost and local clinical audit of outcomes.
by K. Swallow, Dr G. Wild, Dr W. Egner and Dr R. Sargur
Background
Celiac disease is a common autoimmune condition affecting approximately 1 : 100 in the UK [1, 2]. Early diagnosis is key to appropriate management. Symptoms are eliminated by following a gluten-free diet after a confirmed diagnosis. Following best practice guidance can reduce repeated visits to GP practices and outpatient departments, and numerous requests for laboratory tests.
In recent years, guidelines for the diagnosis and management of celiac disease have been published by the National Institute of Health and Care Excellence (NICE) [1] and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) [3]. The guidelines provide algorithms for laboratory testing strategies and recommendations about when a duodenal biopsy should be performed in both symptomatic and asymptomatic patients. There is a perceived need to avoid invasive procedures in children by using optimal in vitro testing.
Guidelines: recommendations for diagnostic testing in symptomatic patients
There are some similarities and several notable differences between the guidelines given for symptomatic patients. Both NICE and ESPGHAN guidance recommend IgA tissue transglutaminase antibodies (TTG Ab) as the first-line screening test. ESPGHAN also recommend testing all patients for IgA deficiency. NICE only recommend checking for IgA deficiency if the IgA TTG Ab result is negative. After this initial test the recommended pathways by the two groups differ; however, both base the strategies on the level of positivity of the IgA TTG Ab result.
NICE pathway after IgA TTG Ab testing
NICE recommend that if the TTG Ab test is positive the patient should be referred for confirmatory biopsy. If it is ‘equivocal’, IgA endomysial antibodies (EMA) should be tested in order to determine if the TTG Ab result is potentially false positive or if the patient should be referred to a gastroenterologist. A patient with negative IgA TTG Ab should be checked for IgA deficiency and IgG TTG/EMA performed if they are found to be deficient. The guidelines do not provide information regarding the definition of the ‘equivocal’ range for TTG Ab assays.
ESPGHAN pathway after IgA TTG Ab testing
For symptomatic patients the diagnosis can be made with or without the need for duodenal biopsy, dependent on the serological test results. As with NICE, all patients with positive IgA TTG Ab should be referred to a gastroenterologist. They also suggest using IgG TTG/EMA if the patient is IgA deficient.
ESPGHAN stratify the level of positivity based upon levels above the normal range or ‘upper limit of normal’ (ULN). If the TTG Ab result is >10× ULN the decision to go to biopsy is made after further testing EMA and HLA-DQ2/DQ8. If all tests (TTG Ab, EMA and HLA-DQ2/DQ8) are positive, a patient may be diagnosed without the need for duodenal biopsy. Biopsy is recommended if EMA and/or HLA typing is negative, or if TTG is positive but <10× ULN.
Guidance for testing in asymptomatic patients (screening)
Several conditions, including IgA deficiency, autoimmune disease (type I diabetes, hypothyroidism, pernicious anemia), Down syndrome, or a having a first degree relative with celiac disease, are associated with an increased risk of the condition. Both sets of guidance recommend that screening should be considered in these groups. NICE testing follows the same pathway as recommended for symptomatic patients. ESPGHAN guidance takes a different approach, recommending HLA-DQ2/DQ8 as the first line test since virtually all celiac cases have these haplotypes. TTG Ab titres are then used to determine if EMA and/or biopsy are required. In this algorithm all patients would need a biopsy to confirm a diagnosis. In contrast NICE state that HLA typing should not be used in initial diagnosis, but can be of use to gastroenterologists in certain cases. Cost of testing will be a factor, as will the availability of an adequate testing strategy for HLA typing.
Laboratory testing for celiac disease: things to consider
Serological tests
The IgA TTG Ab test is an integral part of both published guidelines. This test does not have an international reference preparation, therefore kits available from different manufacturers all perform in a slightly different manner. Monitoring performance of different TTG Ab assays via the UK National External Quality Assessment Service (NEQAS) external quality assurance scheme [4] provided evidence of the lack of consensus between assays from different manufacturers and between laboratories using the same assay. The same sample can generate a range of results when measured as ULNs, with the potential for different pathways being followed depending on the laboratory performing the test. This emphasizes that currently a generic statement about the level of positivity cannot easily be used across the board without local validation of outcomes.
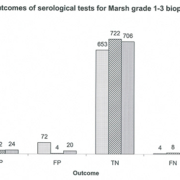
Clinical audit plays an important role in determining whether published guidelines work when there is poor standardization of assays. Audit of your cohort using your assays should be performed. Data published about our experience of serological assay performance when following different testing strategies highlights that false positive IgA TTG Ab results, even at very high titres, can be problematic (Fig. 1) [5]. Other centres have also noted false positive IgA TTG Abs at high levels [6, 7]. Conversely, there are a number of reports that show following ESPGHAN guidance works well [2, 8, 9] and that avoiding biopsy could be reasonably justified in children with high IgA TTG Ab titres.
In all guidelines the EMA test by immunofluorescence is used as a secondary test dependent on the IgA TTG Ab result. Leading to the question about why this test is not used alone if it is being used as a confirmatory check for the TTG Ab test. The EMA test is considered by some to be more expensive, as the laboratory has to have the correct equipment and staff that are competent at performing the test and reading immunofluorescence slides [10] and internal quality control is sometimes more difficult for general laboratories. Alternatively, the IgA TTG Ab assay is an easily automated test that is readily available in non-specialist laboratories on a number of assay platforms. Testing for both TTG and EMA initially in all patients will increase costs with little added benefit [1, 3, 5, 8, 9, 11]. A decision has to be made about which screening test is the most appropriate, both in performance and practicality, for each laboratory. This decision should be made based upon the IgA TTG Ab assay used, so cannot be generalized between laboratories until harmonization of the test is established [5, 9, 11, 12].
Genetic testing
Using HLA-DQ2/DQ8 screening has flaws because of a large false positive population. Individuals with these HLA types are at greater risk of developing celiac disease [3, 8]. Approximately 30% of the healthy Caucasian population have HLA-DQ2 and do not go on to develop the disease [10]. Implementing HLA testing as an initial screening test in asymptomatic children can lead to further testing in some patients that do not have, or will not develop celiac disease. It is also much more expensive than serological tests.
Duodenal biopsy
Duodenal biopsy is considered to be the ‘gold standard’ for diagnosis. However, this is an invasive procedure that is not without risk of complications. Current guidelines explicitly agree that the number of biopsies carried out should be minimized by only performing them in patients that are determined to be at a high risk of having celiac disease following serological tests. There are mixed opinions on whether a diagnosis can be made without the need for biopsy [5, 7, 8, 9, 12]. Duodenal biopsy is the most costly procedure performed during diagnosis of celiac disease. If the numbers of biopsies are reduced, cost savings will be made as well as preventing unnecessary harm in some patients [11].
Best testing strategy?
This depends on your local set-up and audit of outcomes. Current guidelines provide a starting point for determining which tests should be done and when. However the difference in performance between TTG Ab assays has not been adequately recognized. Clinical audit and local validation can help laboratories to decide if it is appropriate to follow the recommended pathways, with the assays that they currently use [5, 7, 8, 9, 11, 12]. This is the only method that can provide a true reflection of the sensitivity, specificity and positive or negative predictive values of the tests locally. This provides an evidence base for justification of the test strategy being used.
Conclusions
The guidelines provide recommendations for the best testing approach but this is not mandatory. A different strategy, for example, using EMA as the first-line screen, could be employed if there is sufficient evidence that this would work better in your laboratory, for your cohort and is economically justified. You must know how your assays perform and assess this using in-house clinical audit and discuss with your local clinicians to provide the best service locally.
The ultimate aim is to provide an approach that will benefit the patient by being the fastest and most reliable method for diagnosis. This relies on selection of the strategy with the best positive and negative predictive values, to avoid biopsies that are not required. Cost is also a major factor in the current economic climate that must be considered when deciding upon the test strategy. It is not currently possible to diagnose celiac disease on the basis of one test result. Choosing the most appropriate strategy for your laboratory can reduce the number of unnecessary referrals and biopsies [11], thereby reducing cost to the healthcare system without an impact on patient care.
References
1. National Institute of Clinical Excellence (NICE) guideline 86. Celiac disease: recognition and assessment of celiac disease, 2009. http://tinyurl.com/q3wspfp
2. Mubarak A, Wolters VM, Gmelig-Meyling FH, Ten Kate FJ, Houwen RH. Tissue transglutaminase levels above 100 U/mL and celiac disease: a prospective study. World J Gastroenterol. 2012; 18(32): 4399–4403.
3. Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, Troncone R, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Paediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of celiac disease. J Pediatr Gastroenterol Nutr. 2012; 54: 136–160.
4. Egner W, Shrimpton A, Sargur R, Patel D, Swallow K. ESPGHAN guidance on celiac disease 2012: multiples of ULN for decision making do not harmonise assay performance across centres. J Pediatr Gastroenterol Nutr. 2012; 55: 733–735.
5. Swallow K, Wild G, Sargur R, Sanders DS, Aziz I, Hopper AD, Egner W. Quality not quantity for transglutaminase antibody 2: the performance of an endomysial and tissue transglutaminase test in screening celiac disease remains stable over time. Clin Exp Immunol; 2013; 171: 100–106.
6. Gidrewicz D, Lyon ME, Trevenen C, Butzner JD. How do the 2012 ESPGHAN celiac disease guidelines perform in a GI clinic. Gastroenterology 2013, 144(5)S1: S-14.
7. Bhardwaj M, Banoub H, Sumar N, Lawson M, Chong S. The impact of ESPGHAN guidelines on the investigations for celiac disease. Arch Dis Child. 2013; 98(Suppl 1): A92.
8. Klapp G, Masip E, Bolonio M, Donat E, Polo B, Ramos D, Ribes-Koninckx C. Celiac disease: the new proposed ESPGHAN diagnostic criteria do work well in a selected population. J Pediatr Gastroenterol Nutr. 2013; 56(3): 251–256.
9. Wolf J, Hasenclever D, Petroff D, Richter T, Uhlig HH, Laaβ MW, Hauer A, et al. Antibodies in the diagnosis of celiac disease: a biopsy controlled, international, multicentre study of 376 children with celiac disease and 695 controls. PLOS One 2014; 9(5): e97853 and Correction PLOS One 2014; 9(8): e105230.
10. Van Heel DA and West J. Recent advances in celiac disease. Gut 2006; 55(7): 1073–1046.
11. Hopper AD, Hadjivassiliou M, Hurlstone DP, Lobo AJ, McAlindon ME, Egner W, Wild G, Sanders DS. What is the role of serologic testing in celiac disease? A prospective, biopsy confirmed study with economic analysis. Clin Gastroenterol Hepatol. 2008; 6: 314–320.
12. Beltran L, Koenig M, Egner W, Howard M, Butt A, Austin MR, Patel D, et al. High titre circulating tissue transglutaminase-2 antibodies predict small bowel villous atrophy, but decision cut-off limits must be locally validated. Clin Expt Immunol. 2014; 176: 190–198.
The authors
Kirsty Swallow* BSc, MSc; Graeme Wild PhD; William Egner PhD, MD, FRCP, FRCPath; and Ravishankar Sargur MD, FRCP, FRCPath
Protein Reference Unit and Immunology Department, Northern General Hospital, Sheffield, UK.
*Corresponding author
E-mail: Kirsty.swallow@sth.nhs.uk