Diagnosis of diabetes mellitus
Diabetes is characterized by hyperglycemia, but diagnosis no longer depends exclusively on plasma glucose measurements. The endorsement of glycated hemoglobin as a diagnostic test for diabetes has seen its widespread adoption for this purpose: it is vital that its application in this role is appropriate and its limitations understood.
by Dr Shirley Bowles
Introduction
The term diabetes mellitus encompasses several diseases of abnormal carbohydrate metabolism that are characterized by hyperglycemia associated with relative or absolute defects in insulin secretion and varying degrees of peripheral resistance to its action [1]. Diabetes is the most common metabolic disorder: in 2014, 422 million people in the world had diabetes, a prevalence of 8.5% in the adult population [2].
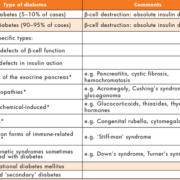
The fact that various pathogenetic processes may be involved in the development of diabetes is illustrated by the etiological classification outlined in Table 1 but, in fact, the vast majority of cases are categorized as either Type 1 (5–10%) or Type 2 (90–95%). Type 1 diabetes is usually due to cellular-mediated autoimmune destruction of the pancreatic β-cells, with absolute loss of insulin secretion, and, although the rate of cell destruction is variable, most individuals will ultimately become dependent on exogenously administered insulin for survival, and are at risk of ketoacidosis. In contrast, patients with Type 2 diabetes often have insulin levels that appear normal, or even elevated, but secretion is considered defective because it is insufficient to compensate for varying degrees of insulin resistance, which may be attributable to the obesity found in most of these patients. In Type 2 diabetes, treatment with insulin is not essential for survival, although it may eventually prove necessary to achieve glycemic control [3].
The majority of individuals with Type 2 diabetes are largely asymptomatic, diagnosed only after laboratory evaluation, whereas those with Type 1 are more likely to present with the classical symptoms of hyperglycemia: polyuria, polydipsia, blurred vision and weight loss. There may also be acute life-threatening consequences of uncontrolled diabetes: diabetic ketoacidosis in Type 1 diabetes and non-ketotic hyperosmolar syndrome in Type 2 [1]. Both forms are associated with a number of characteristic long-term complications, usually considered to be a consequence of microvascular disease, including retinopathy, with potential loss of vision; nephropathy, leading to kidney failure; peripheral and autonomic neuropathy. However, in reality, the major determinant of the reduced life expectancy seen in diabetes is the significantly increased incidence of macrovascular atherosclerotic disease, which causes myocardial infarction or angina, stroke or peripheral vascular disease [4].
Diagnostic criteria
1. Blood glucose measurements
For decades, the diagnosis of diabetes was based exclusively on glucose measurements but, as blood glucose is a continuous variable, cut-off points for diagnosis are necessarily somewhat arbitrary, and information derived from research and clinical practice has prompted periodic re-evaluation of the diagnostic criteria. By 1997, the diagnosis of diabetes, as defined by the World Health Organization (WHO), required a fasting plasma glucose (FPG) of ≥7.8 mmol/L or a plasma glucose (PG) of at least 11.1 mmol/L, in either a random blood specimen or in one collected 2 hours after a standard 75-g glucose load, as part of an oral glucose tolerance test (OGTT). An ‘at-risk’ category was also recognized: impaired glucose tolerance (IGT), which was identified on the basis of an OGTT 2-hour PG of 7.8–11.0 mmol/L. These values were chosen, based on the risk of future symptoms of uncontrolled hyperglycemia [5].
As the major objective of diagnosing diabetes is to intervene so as to prevent premature mortality and morbidity, it seemed logical to consider diagnosis in terms of risk of complications: following the recommendations of the National Diabetes Data Group in 1997 [6], the WHO revised the diagnostic threshold, with respect to the fasting glucose, based on the observed association between glucose levels and the risk of developing the microvascular complication of retinopathy. The OGTT 2-hour PG of ≥11.1 mmol/L closely approximates to a point at which the prevalence of microvascular complications increases dramatically. However, only approximately 25% of those who exceed this 2-hour threshold will also have a FPG ≥7.8 mmol/L, whereas almost all individuals with FPG ≥7.8 mmol/L have a 2-hour OGTT level ≥11.1 mmol/L. Thus, this earlier FPG cut-off defined a greater degree of hyperglycemia, a discrepancy that was considered undesirable: both fasting and 2-hour cut-off points should reflect a similar degree of hyperglycemia and risk of adverse outcomes. In addition, due to the inconvenience of undertaking OGTTs, the FPG alone was often performed, meaning that a substantial number of individuals, who were at increased risk of microvascular complications, would not have been detected. The revised FPG cut-off of ≥7.0 mmol/L was shown to have a similar predictive value for adverse outcomes as the 11.1 mmol/L 2-hour OGTT threshold, which validated the use of this simpler test for diagnostic purposes.
It was at this stage that a secondary criterion for the ‘at-risk’ category was recognized: impaired fasting glycemia (IFG), a FPG of 6.1–6.9 mmol/L. Both IFG, and the previously described IGT, have been referred to as ‘pre-diabetes’, indicating a relatively high risk of future diabetes. Studies have demonstrated an approximately 5–10% annualized risk of progression to diabetes in individuals with either IFG or IGT and 10–15% in those with both abnormalities [7].
2. Glycated hemoglobin
Glycated hemoglobin (HbA1c), formed as a consequence of a non-enzymatic, irreversible reaction between glucose and the N-terminal valine residue of the β globin chains of hemoglobin, reflects average blood glucose levels over the preceding 8–12-week period (the lifespan of a red blood cell) and its potential as an indicator of glycemic control was recognized in 1977 [8]. Over the intervening years, supported by evidence from the Diabetes Control and Complications Trial (Type 1 diabetes) [9] and the United Kingdom Prospective Diabetes Study (Type 2 diabetes) [10], which validated the direct relationship between glycated hemoglobin levels and clinical outcomes, it has had a vital role in monitoring diabetes. With respect to the diagnosis of diabetes, however, although epidemiological studies also showed a clear relationship between HbA1c and retinopathy, variation in methodology and standardization, and concern about the confounding effect of factors affecting erythrocyte turnover, seemed to preclude its use for this purpose [8]. This situation has changed in recent years, as a result of a number of HbA1c standardization programmes, culminating in the work of the IFCC Working Group on Standardization of HbA1c, which established true International Reference Methods for HbA1c and provided a preparation of pure HbA1c, against which manufacturers could standardize their calibrators [11].
In 2011, in response to this global standardization of HbA1c methods, the WHO stated that HbA1c could be used as a diagnostic test for diabetes mellitus, “provided that stringent quality assurance methods are in place, assays are standardized to criteria aligned to the international reference values and there are no conditions present that preclude its accurate measurement” [12]. Based on the DETECT-2 pooled data analysis, which examined the association between diabetes-specific retinopathy and glycemic measures, an HbA1c of 48 mmol/mol was recommended as the cut-off point for diagnosing diabetes [13]. As with glucose measurements, there is a range of HbA1c levels below this diagnostic value, which indicates an increased risk of future diabetes and/or cardiovascular disease: a systematic review indicated that HbA1c values between 37 and 48 mmol/mol are associated with a substantially increased risk of diabetes [14]. The WHO did not provide specific guidance on HbA1c criteria for ‘pre-diabetes’ but the 2009 International Expert Committee concluded that individuals with an HbA1c of 42–47 mmol/mol should be considered at high risk of progression to diabetes [15] (estimated 5-year risk of 25–50% [14]), a range that was endorsed by a UK Expert Position Statement [16].
Current recommendations
The current criteria for the diagnosis of diabetes and ‘pre-diabetes’, in accordance with WHO recommendations, are summarized in Table 2. OGTTs, which are time-consuming, inconvenient and show poor reproducibility, are increasingly confined to the diagnosis of gestational diabetes. HbA1c confers definite advantages over FPG (and OGTT): no patient preparation; lower biological variation; less fluctuation in acute stress and illness, and standardization of measurement is now better than for glucose, which has no internationally recognized reference method. However, there are a number of situations, in which the use of HbA1c for diagnosis is not appropriate (Table 2): as a measure of chronic hyperglycemia, HbA1c should not be used where rapidly developing hyperglycemia is suspected and results will be unreliable in the presence of any factors affecting erythrocyte lifespan [12].
Regardless of the test used, in an asymptomatic patient, a diagnostic result should be confirmed by repeat testing on a separate day, preferably using the same test, in order to increase the likelihood of concordance. In the same way that there is less than 100% concordance between the results of FPG and 2-hour OGTT PG, there is not full concordance between HbA1c and glucose measurements: these three different measures of glycemia represent different physiological processes and, therefore, inevitably, they identify somewhat different populations of patients [17]. In fact, although HbA1c performs equally well as a predictor of retinopathy risk, in most populations, its use results in a lower diabetes prevalence (the OGTT 2-hour PG is the most sensitive test). A study including 6890 adults from the US National Health and Nutrition Examination Survey (1999–2006) indicated that the prevalence of undiagnosed diabetes was 2.3% using HbA1c, compared to 3.6% using FPG [18]. Other studies have confirmed this discrepancy although, in fact, the magnitude of the difference appears to vary between populations, perhaps reflecting geographical or ethnic differences in hemoglobin glycation rates or the distribution of certain forms of anemia or hemoglobinopathy. It is anticipated that, in practice, the lower sensitivity of HbA1c will be mitigated by its ease of use, which will facilitate its wider application [3].
For those individuals with ‘pre-diabetes’, structured lifestyle intervention, aimed at increasing physical activity and achieving a loss of body weight, may prevent, or at least delay, the development of diabetes. Within this category, for all three tests, the risk of future diabetes is curvilinear, extending below the lower limit of the range and becoming disproportionately greater at the higher end: accordingly, intervention and follow-up should be most aggressive for those considered at particularly high risk [3]. The associated increased risk of cardiovascular disease should also be targeted, with appropriate management of other relevant risk factors (smoking, lipids, blood pressure).
From glucose measurements to HbA1c in the diagnosis of diabetes mellitus: one UK laboratory’s experience of the change in clinical practice
Guidance, outlining the WHO’s position on the use of HbA1c in the diagnosis of diabetes, was issued to local clinicians in 2012. Subsequently, in September 2014, updated guidance was disseminated, advocating the use of HbA1c as a diagnostic test for diabetes mellitus, except where inappropriate, and providing advice on follow-up. This was supported by modification of the requesting process, which allowed a distinction to be made between HbA1c requests made for monitoring established diabetes (designated HbA1cM) and those being used for diagnosis (designated HbA1cD). This facilitated the provision of additional targeted guidance in the form of interpretative comments and, importantly, for HbA1cD requests, allowed flagging, as abnormal, results that indicated ‘pre-diabetes’ (42–47 mmol/mol).
The pattern of fasting glucose, OGTT (excluding those from maternity services) and HbA1c requesting between April 2012 and March 2016 is summarized in the Figure 1. Between late 2012 and September 2014, there was a steady increase in HbA1c requests, which was mirrored by a decrease in the number of fasting glucoses requested and OGTTs performed. Since the introduction of the two separate requests, HbA1cD and HbA1cM, in September 2014, it can be seen that, with regard to monitoring, the number of requests has remained at around 2200 per month, about 10% higher than the number being done early in 2012 (when all such requests were for this purpose). In contrast, those requested for diagnostic purposes increased rapidly and, since late 2015, the number of HbA1cD requests has been similar to the total number of HbA1c requests/month in 2014.
Summary
Local experience indicates an enthusiastic uptake in the use of HbA1c for diagnosing diabetes and a concurrent fall in glucose measurements (FPG and 2-hour OGTT PG) for this purpose. As anticipated, the convenience of this test has led to increased screening for diabetes but there is concern that this ease of use may mean that the limitations of HbA1c as a diagnostic test are overlooked, resulting in its application in circumstances when glucose measurements would, in fact, be indicated. There is a clear role for laboratory staff in the provision of ongoing education of clinicians, in order to ensure the appropriate use and interpretation of these tests.
References
1. McCulloch DK. Clinical presentation and diagnosis of diabetes mellitus in adults. UpToDate. (http://uptodate.com/contents/clinical-presentation-and-diagnosis-of-diabetes-mellitus)
2. Global Report on Diabetes. World Health Organization 2016. (http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf)
3. American Diabetes Association Position Statement. Diagnosis and classification of diabetes mellitus. Diabetes Care 2011; 34(Suppl 1): S62–S69.
4. Report of a WHO Consultation. Definition, diagnosis and classification of diabetes mellitus and its complications. World Health Organization 1999. (https://www.staff.ncl.ac.uk/philip.home/who_dmg.pdf)
5. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. World Health Organization 2006. (http://www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf)
6. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997; 20: 1183–1197.
7. Inzucchi SE. Diagnosis of diabetes. N Engl J Med. 2012; 367(6): 542–550.
8. Day A. HbA1c and diagnosis of diabetes. The test has finally come of age. Ann Clin Biochem. 2012; 49: 7–8.
9. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes. N Engl J Med. 1993: 329: 977–986.
10. United Kingdom Prospective Diabetes Study (UKPDS) Group. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853.
11. The American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and Laboratory Medicine and the International Diabetes Federation Consensus Committee. Consensus statement on the worldwide standardisation of the HbA1c measurement. Diabetologia 2007; 50(10): 2042–2043.
12. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. Abbreviated report of a WHO consultation. World Health Organization 2011. (http://www.who.int/diabetes/publications/report-hba1c_2011.pdf)
13. Colagiuri S, Lee CMY, Wong TW, Balkau B, Shaw JE, Borch-Johnsen K. Glycemic thresholds for diabetes-specific retinopathy: implications for diagnostic criteria for diabetes. Diabetes Care 2011; 34: 145–150.
14. Zhang X, Gregg EW, Wiliamson DF, Barker LE, Thomas W, Imperatore G, Williams DE, Albright AL. A1c level and future risk of diabetes: a systematic review. Diabetes Care 2010; 33(7): 1665–1673.
15. International Expert Position Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009; 32: 1327–1334.
16. Expert Position Statement: Use of HbA1c in the diagnosis of diabetes mellitus in the UK. The implementation of World Health Organization guidance 2011. Diabetic Medicine 2012; 29: 1350–1357.
17. American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 2015; 38(Suppl 1): S8–S16.
18. Carson AP, Reynolds K, Fonseca VA, Muntner P. Comparison of A1C and fasting glucose criteria to diagnose diabetes among U.S. adults. Diabetes Care 2010; 33: 95–97.
The author
Shirley A. Bowles MB ChB, MSc, FRCPath
Department of Blood Sciences, Countess of Chester Hospital NHS Foundation Trust, Chester, UK
E-mail: shirleybowles@nhs.net