Diagnosis work-up of iron deficiency anemia in patients with inflammatory bowel disease
Iron deficiency and anemia are common side-effects of inflammatory bowel disease. Analysis of a number of components of the iron-metabolism pathway can aid a differential diagnosis.
by Professor Jürgen Stein
Iron deficiency occurs in about 60–80% of patients with inflammatory bowel disease (IBD), and anemia manifests in approximately one-third of patients. Anemia is thus by far the most common extraintestinal complication of IBD. In a recent review by Gisbert and Gomollón, study data showed the prevalence of anemia in patients with IBD to range from 16% to 74%, with a mean value of 16% in outpatients and 68% in hospitalised patients [1]. Goodhand et al. demonstrated in a more recently-published prospective trial that anemia and iron deficiency anemia (IDA) are particularly prevalent in children, the incidence of anemia being 70% in children, 42% in adolescents, and 40% in adults [2]. Iron deficiency was also found to occur more commonly in children (88%) and adolescents (83%) than in adults (55%).
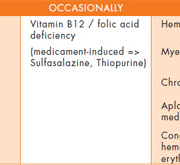
The cause of anemia in patients with IBD is multifactorial [Table 1]. The two most frequent etiological forms by far are IDA (resulting from iron deficiency secondary to blood loss through the ulcerations of the intestinal mucosa, reduced iron absorption and reduced intake) and anemia of chronic disease (ACD), described for the first time by Cartwright in 1946 [3]. ACD is characterised by normal or reduced mean corpuscular volume (MCV), reduced serum iron, reduced total iron binding capacity (TIBC), normal to elevated serum ferritin level, and reticuloendothelial system (RES) stores that are elevated relative to total body iron. While vitamin B12-folate deficiency and drug-induced anemia (sulfasalazine, thiopurines, methotrexate, calcineurin inhibitors) are less widespread, these possibilities should also be considered (for extended reviews
see Stein and Diagnass 2010, 2011, 2012 [4–6]).
Depending on severity, it is differentiated into three stages: (I) depleted iron stores, (II) functional iron deficiency with iron-deficient erythropoiesis, and (III) iron deficiency anemia.
Stage I depletion of iron stores is not associated with functional problems. It is only upon transition to stage II (iron-deficient erythropoiesis) that iron deficiency becomes a disorder because the cells can no longer be adequately supplied with iron. In stage III, the deficient iron supply to the body’s cells is already so pronounced that hemoglobin concentrations fall below the normal range.
In principle, all compartments of the body’s iron metabolism can be conveniently monitored with routine laboratory methods:
• Iron stores: serum ferritin
• Iron transport: transferrin saturation
• Iron utilisation with erythropoiesis: e.g. proportion of hypochromic erythrocytes or reticulocytes
Serum iron concentrations are governed by a circadian rhythm and can be low even in cases of anemia of chronic disease (ACD). Its role in the work-up of iron deficiency is, therefore, obsolete.
Serum ferritin
Serum ferritin is an indicator for the iron stores contained in the reticulohistiocytic system. Determining the serum ferritin concentration serves to identify disorders of the cellular iron stores (total body iron stores). The reference range for women is 15–100 μg/L and 30–200 μg/L for men; a serum ferritin concentration of 100 μg/L represents about 1000 mg of stored iron. Reduced concentrations are a sign of iron deficiency: a serum ferritin concentration <15 μg/L is considered a sign of an absolute iron deficiency. Because both ferritin and transferrin belong to the family of acute-phase proteins (APP), these reference ranges do not apply to patients with active inflammatory bowel disease.
In the context of inflammatory processes and the increased release of ferritin from damaged tissue, there may be an increase in serum ferritin levels. Hence, patients who actually suffer from iron deficiency will appear to have a normal iron status. In such cases, ferritin concentrations of 15 (30)–100 μg/L should be considered suspicious for iron deficiency. The differential diagnosis should, therefore, be based on serial measurements of inflammation parameters that are independent of iron metabolism [erythrocyte sedimentation rate (ESR), CRP].
Transferrin/transferrin saturation
Disorders of iron transport can be identified by determining the transferrin concentration. Iron deficiency is usually associated with a reduced transferrin saturation (TSAT). TSAT, expressed in per cent, is the quotient of the iron concentration (μmol/L) divided by the transferrin concentration (mg/dL) in serum or plasma multiplied by 70.9 (fasting blood sample).
Transferrin saturation is a measure for the iron load of circulating transferrin, the plasma protein responsible for transporting iron from its storage site to the bone marrow. Thus, determination of transferrin saturation does not provide any information regarding the status of the iron stores and provides only an indirect indication of the extent of iron utilisation in the bone marrow. Under physiological conditions, 16–45% of transferrin molecules in plasma are “loaded” with iron (3–4 mol of iron per mol of transferrin). Saturations <16% are considered to represent a suboptimum iron supply for the erythropoietic process. A reduced transferrin saturation (<20%) is associated with a relatively good sensitivity (90%) for recognising iron deficiency states, with, however, only a relatively low specificity (40–50%). Because the measurement of serum iron and serum transferrin are both subject to fairly significant circadian effects, blood samples should always be obtained at the same time of day and repeated frequently. Serum transferrin levels are increased in patients taking oral contraceptive steroids and reduced with inflammation (negative APP), meaning that, in patients with acute or chronic inflammatory disorders, TSAT may be reduced despite normal iron stores.
Soluble transferrin receptor
While all cells in the body are supplied with transferrin receptors, the bulk of these (80%) are found in the bone marrow. The number of transferrin receptors on the cell surface is an indicator for that cell’s iron requirements. In cases of functional iron deficiency, i.e. inadequate availability of iron for normal erythropoiesis, the number of receptors on the cell membrane is up regulated. Because the transferrin receptors are continuously shed from the cell membrane and pass into the plasma as soluble transferrin receptors (sTfR), the serum concentration of sTfR serves as an indicator of iron supply for erythropoiesis. TfR is up regulated in iron deficiency. In contrast to ferritin and transferrin, neither chronic inflammation nor liver damage has any effect on TfR. Elevated concentrations of sTfR are found in iron deficiency as well as every other expansion of erythropoiesis, including haemolytic anemia, the thalassemias and polycytemias. Conversely, sTfR concentrations are reduced in aplastic anemia and other conditions characterised by hypoproliferative erythropoiesis, such as renal anemia.
TfR-F index
The sensitivity and specificity of sTfR as a parameter for assessing iron-deficient erythropoiesis can be enhanced by the parallel determination of sTfR and ferritin, which can then be used to calculate the so-called TfR-F index. The TfR-F index is defined as the quotient of the concentration of sTfR (mg/L) and log serum ferritin (μg/L). This quotient represents a marker that is dependent on the status of the iron stores, the availability of iron for erythropoiesis as well as the erythropoietic activity. In individuals with a deficiency of iron stores, the TfR-F index is increased. Disadvantageous for the routine diagnostic use of the TfR-F index are its lack of uniform reference range (the reference ranges of the
individual components are assay-dependent) and the relatively high costs.
Hypochromic erythrocytes/reticulocyte hemoglobin
Determination of the cellular hemoglobin content of reticulocytes (CHr) and the proportion of hypochromic red cells (%HYPO) is a valuable marker in the temporal differential diagnosis of iron deficiency anemia. Because the maturation time for reticulocytes is 3–5 days in the bone marrow and 1 day in the peripheral blood, the drop in CHr represents a marker for current iron deficiency. By contrast, a decline in %HYPO, because it is dependent of the normal red cell life span of 120 days, reflects longer-standing iron deficiencies. Thus, CHr and %HYPO can be considered analogous to blood glucose and HgA1C determinations in diabetics.
Some blood count units (Adiva-120, Technicon H1, H2 und H3; Bayer, Leverkusen, Germany) have the capability, without significant additional expense, to measure the hemoglobin content of each individual erythrocyte and calculate the proportion of hypochromic red cells while at the same time assessing reticulocytes for their volume and hemoglobin content. In people without iron deficiency and in those in stage I, the proportion of hypochromic red cells (hemoglobin content <28 pg) is less than 2.5%. Values >10% are considered confirmatory for iron deficient erythropoiesis. The increase in %HYPO precedes microcytic changes in the blood count. CHr values <26 pg are also considered confirmatory for iron-deficient erythropoiesis.
Zinc protoporphyrin
A deficiency in available iron for erythropoiesis leads to a compensatory incorporation of zinc into the protoporphyrin complex [Figure 1], and the increased formation of zinc protoporphyrin (ZPP), because of its relatively strong fluorescence in whole blood, is easily measured using HPLC-coupled fluorescence detection. Individuals with iron store deficiency exhibit normal ZPP values as long as the erythropoietic process is adequately supplied with iron. The onset of iron-deficient erythropoiesis triggers continuously increasing ZPP concentrations. Concentrations <40 μmol/mol haeme are considered normal. Values of 40–80 μmol/mol haeme represent latent iron deficiency (hemoglobin normal); >80 μmol/mol haeme are associated with manifest iron deficiency. In severe cases, values up to 1000 μmol/mol haeme have been reported. Thus, ZPP determination not only recognises iron-deficient erythropoiesis but also quantifies it.
Hepcidin
The regulation of iron homeostasis in IDA, ACD and ACD/IDA involves the iron regulatory protein hepcidin, a type II acute phase protein. During inflammation, interleukin (IL) 6 induces hepcidin production which leads to a decrease in dietary iron absorption and macrophage iron release, leading to decreased circulating iron and impaired iron distribution within the body. Based on previous studies carried out in rats and humans showing elevated hepcidin-25 levels in ACD individuals and intermediate levels in ACD/IDA, it was assumed that measuring serum hepcidin levels could help differentiation between ACD and ACD/IDA.
Abbreviations
%HYPO, proportion of hypochromic red cells; APP, acute phase proteins; ACD, anemia of chronic disease; CHr, cellular hemoglobin content of reticulocytes; CRP, C-reactive protein; Hb, hemoglobin; IBD, inflammatory bowel disease; IDA, iron deficiency anemia; MCV, mean corpuscular volume; TSAT, transferrin saturation; (s)TfR, (soluble) transferrin receptor; ZPP;
zinc protoporphyrin.
References
1. Gisbert JP, Gomollon F. Common misconceptions in the diagnosis and management of anemia in inflammatory bowel disease. Am J Gastroenterol 2008; 103(5): 1299–1307.
2. Goodhand JR, Kamperidis N, Rao A, Laskaratos F, McDermott A, Wahed M, Naik S, Croft NM, Lindsay JO, Sanderson IR and others. Prevalence and management of anemia in children, adolescents, and adults with inflammatory bowel disease. Inflamm Bowel Dis 2012; 18(3): 513–519.
3. Cartwright GE, Lauritsen MA, Humphreys S, Jones PJ, Merrill I M, Wintrobe MM. The anemia associated with chronic infection. Science 1946; 103(2664): 72–73.
4. Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol 2010; 7: 599–610.
5. Stein J, Dignass A. Management of Iron Deficiency Anemia in Inflammatory Bowel Disease with Special Emphasis on Intravenous Iron. Practical Gastroenterol 2011; 35: 17–30.
6. Stein J, Dignass A. Management of iron deficiency anemia in inflammatory bowel disease – a practical approach. Ann Gastroenterol 2012, in press.
The author
Jürgen Stein MD, PhD
Crohn Colitis Clinical Research Center Rhein-Main
Frankfurt/Main, Germany
E-mail: J.Stein@em.uni-frankfurt.de