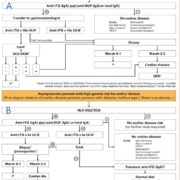
Diagnostic strategy for coeliac disease in line with new ESPGHAN guidelines
Newly revised guidelines for the diagnosis of coeliac disease (CD) place greater emphasis on laboratory testing, enabling the number of small-intestinal biopsies performed to be significantly reduced. The detection of antibodies against tissue transglutaminase (anti-tTG) or endomysium (EmA) remains a cornerstone of diagnosis, while further diagnostic procedures have gained new significance. The molecular genetic determination of the human leukocyte antigens (HLA) DQ2 and DQ8 now plays a central role in diagnosis, thanks to a better understanding of the genetic factors underpinning the disease. Moreover, state-of-the-art assays for antibodies against deamidated gliadin peptides (DGP), as oppose to native gliadin, now constitute a highly sensitive and specific analysis to support diagnosis. In the new guidelines, anti-tTG and anti-DGP are recommended as first-line tests in symptomatic individuals, while HLA-DQ2/DQ8 analysis is the initial step for screening asymptomatic persons with a high disease risk.
by Dr Jacqueline Gosink
CD, which is also known as gluten-sensitive enteropathy or non-tropical sprue, is an autoimmune disease caused in genetically predisposed individuals by consumption of gluten-containing cereals. The disease process is triggered by protein components of gluten known as prolamins, of which gliadin is the most common. Partially digested prolamin peptides are chemically modified (deamidated) in the intestine wall by the enzyme tTG. The immune system of genetically predisposed persons reacts with both the deamidated peptides and tTG, causing chronic inflammation of the small-intestinal mucosa, which results in atrophy of the villi and reduced resorption of nutrients. The only effective treatment for CD is observance of a gluten-free diet.
A clinical chameleon
The classic symptoms of CD are fatigue, abdominal pain, diarrhoea, effects of malabsorption such as weight loss, anaemia and growth retardation in children, vomiting, constipation and bone pains. However, CD is now recognised to be a multifaceted condition which can manifest in many ways. Some patients have non-typical symptoms such as osteoporosis, neuropathies, carditis, pregnancy problems or lymphoma. CD patients may also suffer from Duhring’s dermatitis herpetiformis, a recurrent skin disease characterised by subepidermal blisters.
The disease may also present in silent, latent or potential forms [1]. In the silent form, patients are asymptomatic, but nevertheless exhibit CD-specific antibodies, relevant HLA alleles and villous atrophy. Those with latent CD have previously had a gluten-dependent enteropathy, but are now free of enteropathy; they may or may not exhibit antibodies and/or symptoms. In cases of potential CD, individuals have positive antibodies and compatible HLA, but as yet no symptoms; they may or may not go on to develop CD.
While the prevalence of symptomatic CD is around 0.1%, the prevalence of the disease in all its forms is estimated to be as high as 1%. Many experts now speak of the coeliac disease iceberg, in which classic CD represents only the tip.
New ESPGHAN diagnostic criteria
Early in 2012, the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) released a revised version of its 1990 guidelines for the diagnosis of coeliac disease [1], which were compiled by a group of 17 international experts in the field. The new diagnostic criteria are defined by two algorithms: algorithm 1 [Figure 1A] is applied to symptomatic individuals, while algorithm 2 [Figure 1B] is used for asymptomatic individuals with a high disease risk, for example first-degree relatives of CD patients and patients with type 1 diabetes mellitus, Down’s syndrome, autoimmune thyroid or liver disease, Turner’s syndrome, Williams’ syndrome or selective IgA deficiency.
In algorithm 1 the first-line approach is the determination of anti-tTG antibodies of class IgA in patient serum. In order to exclude the possibility of an IgA deficiency, either total IgA or specific IgG (e.g. deamidated gliadin) should be investigated in parallel. If the anti-tTG antibody titre is very high (>10 times upper normal limit), and if this result is reinforced by positive EmA and compatible HLA, it is no longer considered necessary to perform a biopsy. If serological/genetic findings are inconclusive, results must be confirmed by histological examination of duodenal biopsy tissue to demonstrate villous atrophy and crypt hyperplasia. Diagnostic tests should be carried out in individuals on a gluten-containing diet. A gluten challenge is now only performed under exceptional circumstances.
In algorithm 2, the genetic parameters HLA-DQ2/DQ8 are initially determined to establish the genetic susceptibility. If these are negative, the risk of CD is negligible and no further tests are required. If HLA alleles are compatible with CD, specific antibody tests are used to follow up. In this group a duodenal biopsy is a prerequisite for a definite diagnosis of CD.
The individual diagnostic parameters and the technologies used to detect them are reviewed in the following sections.
Antibodies against tissue transglutaminase (anti-tTG, EmA)
Autoantibodies against tTG of immunoglobulin class IgA are the most important serological marker for CD, as they possess a very high sensitivity and specificity for the disease. While they are virtually never found in healthy individuals or patients with other intestinal diseases, their prevalence in untreated CD is near to 100%. Anti-tTG antibodies are alternatively known as EmA, depending on the test method used: EmA are determined using indirect immunofluorescence [Figure 2], while anti-tTG are detected using monospecific test systems such as ELISA [Figure 3].
Detection of EmA using indirect immunofluorescence is considered the reference standard for CD-specific antibodies due to its unsurpassed sensitivity and specificity. However, the microscopic evaluation required is demanding and dependent on the proficiency of laboratory staff. Enzyme immunoassays for detection of anti-tTG antibodies are often preferred due to their simplicity, cost-effectiveness and automatability, combined with their high sensitivity and specificity. Modern ELISAs for determination of anti-tTG antibodies are based on recombinant human tTG. A multitude of clinical studies have confirmed the efficacy of this method, with high-quality tests yielding a sensitivity of 90-100% and a specificity of 95-100% for active CD.
Antibodies against deamidated gliadin peptides (DGP)
Antibodies against DGP have recently assumed a more important diagnostic role, due to the development of highly sensitive and specific test systems to detect them. Conventional assays based on native full-length gliadin, which frequently yield unspecific reactions with sera from healthy persons, are now obsolete.
The advances in test design were precipitated after research revealed that only a tenth of the epitopes of the gliadin molecule are diagnostically relevant, and these must be present in deamidated form [2]. Based on these observations a novel recombinant gliadin-analogous fusion peptide (GAF) consisting of two nonapeptide components expressed in trimeric form (3X) was created [Figure 4]. The remaining 90% of the molecule was omitted, as it serves predominantly as a target for unspecific reactions.
This designer fusion protein is now used as the target antigen in the Anti-Gliadin (GAF-3X) ELISA, which provides vastly superior performance compared to conventional anti-gliadin ELISAs [3, 4]. In a multicentre study using a total of over 900 sera, the new test yielded a sensitivity (at 95% specificity) of 83%/94% (IgA/IgG) compared to 54%/31% for a conventional anti-gliadin ELISA. This represents an increase of 29% for IgA and 63% for IgG, significantly enhancing the relevance of the analysis.
Use of the Anti-Gliadin (GAF-3X) ELISA in combination with the Anti-tTG ELISA significantly increases the serological detection rate for CD and dermatitis herpetiformis [5]. The IgG version of the ELISA is particularly valuable for identifying CD patients with an IgA deficiency [6], which is frequently associated with CD. Determination of antibodies against DGP is also suitable for assessing disease activity and for monitoring a gluten-free diet or a gluten-load test.
HLA-DQ2 and DQ8
HLA-DQ2 and DQ8 are the principle determinants of genetic susceptibility for CD and are found in virtually all patients. The strong genetic background to CD is highlighted by familial prevalences of 10% in first-degree relatives of patients, 70% in identical twins and 11% in non-identical twins. However, the presence of HLA-DQ2/DQ8 is not sufficient by itself to cause CD. Around a third of the healthy population exhibits DQ2/DQ8 alleles.
Although not a particularly specific parameter, HLA-DQ2/DQ8 is a valuable tool for exclusion diagnostics. If neither DQ2 or DQ8 are present, then CD can be virtually ruled out. It is for this reason that DQ2/DQ8 analysis is now recommended as the first-line test for screening asymptomatic persons at high risk of CD, as defined by the presence of an associated disease or family history (algorithm 2). If DQ2/DQ8 is negative no further follow up is necessary. HLA-DQ2/DQ8 also functions as a confirmatory parameter in symptomatic persons (algorithm 1), and it is one of a triad of laboratory parameters that can be employed to diagnose CD without biopsy in these individuals. DQ2/DQ8 analysis is also helpful for clarifying cases in which diagnosis is inconclusive due to ambiguous serological/biopsy results, especially in infants or in patients who are already on a gluten-free diet, and for differentiation of CD from other intestinal diseases.
HLA-DQ2/DQ8 alleles can be determined using microarray test systems such as the EUROArray system. This analysis is simple to perform, requiring no previous knowledge of molecular biology. Disease-associated gene sections are amplified from purified genomic patient DNA samples by the polymerase chain reaction (PCR) [Figure 5]. The fluorescently labelled PCR products are then detected using microarray BIOCHIP slides composed of immobilised complementary probes. The evaluation [Figure 6] and documentation of results is fully automated using specially developed software (EUROArrayScan). In clinical studies employing precharacterised samples, this microarray yielded a sensitivity of 100% and a specificity also of 100% [7], demonstrating its ability to deliver accurate and reliable results in HLA analysis.
Conclusions
The publication of updated ESPGHAN guidelines for the diagnosis of coeliac disease has reinforced the indispensible role of anti-tTG and EMA in diagnosis and propelled further laboratory diagnostic parameters such as HLA-DQ2/DQ8 and anti-DGP into the limelight. In clear-cut cases, a thorough serological and genetic investigation is now considered sufficient to obtain a diagnosis, allowing the costs and patient discomfort associated with biopsy to be avoided. The state-of-the-art diagnostic tools available today are not only a boon for patient diagnosis, but will also help to advance our understanding of this enigmatic and seemingly widely occurring disease.
References
1. Husby S et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition Guidelines for the diagnosis of CD. JPGN 2012; 54: 136–160.
2. Schwerz E et al. Serologic assay based on gliadin-related nonapeptides as a highly sensitive and specific diagnostic aid in celiac disease. Clin Chem 2004; 50: 2370-2375.
3. Prause C et al. Antibodies against deamidated gliadin as new and accurate biomarkers of childhood CD. JPGN 2009; 49: 52-58.
4. Prause C et al. New developments in serodiagnosis of childhood celiac disease. Ann NY Acad Sci 2009; 1173: 28-35.
5. Kasperkiewicz M et al. Novel assay for detecting celiac disease-associated autoantibodies in dermatitis herpetiformis using deamidated gliadin-analogous fusion peptides. J Am Acad Dermatol [Epub ahead of print] (2011).
6. Villalta D et al. IgG Antibodies against deamidated gliadin peptides for diagnosis of celiac disease in patients with IgA deficiency. Clin Chem 2010; 56: 464-468.
7. Pfeiffer T et al. Microarray based analysis of the genetic risk factors HLA-DQ2/DQ8 – a novel test system for the diagnostic exclusion of celiac disease. 44th Annual Meeting of The European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN), Italy, May 2011.
The author
Dr Jacqueline Gosink
EUROIMMUN AG
Seekamp 31
23560 Luebeck
Germany
Tel: +49 451 5855 25881
e-mail: j.gosink@euroimmun.de