Diagnostic testing for Clostridium difficile: where to go from here?
Clostridium difficile causes serious life-threatening infections but this organism has a complex pathogenesis that makes differentiating true infection from asymptomatic carriage difficult. There are a number of diagnostic testing approaches that can be used alone or in multi-step algorithms. This review discusses the impact that the type of diagnostic test has on interpretation of clinically significant infection, initiation of treatment of C. difficile infection, and how future diagnostic testing may need to differentiate asymptomatic carriage from clinically significant disease.
by Dr Michelle J. Alfa
Clostridium difficile pathogenesis
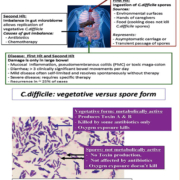
Since the initial report by Bartlett et al. in 1978 [1] that C. difficile could cause infectious diarrhoea in patients who were treated with antibiotics (in particular clindamycin), there have been significant changes in the understanding of the pathogenesis of this organism as well as the approach to the diagnosis of the illness it causes. Initially, C. difficile was thought to be solely a hospital-acquired infection associated with a history of antibiotic consumption. Subsequently it became clear that humans can have toxigenic C. difficile present asymptomatically in their gastrointestinal tract and, unlike other enteric pathogens, the concept of ‘infectious dose’ does not really apply to this gastrointestinal pathogen. C. difficile infection (CDI) is a ‘two-hit’ process (Fig. 1). The first hit is ingestion of the metabolically inactive spore form which does not produce toxin, and the second hit is an imbalance of the gut microbiome (most often due to antibiotic therapy that eradicates gut normal flora without killing the spore or vegetative form of C. difficile). These two hits allow the ingested spore to germinate in the gut to the vegetative form which then replicates and produces Toxin A (enterotoxin) and Toxin B (cytotoxin). These toxins work synergistically to cause mucosal inflammation in the colon and diarrhoea (the small intestine is not damaged). Although the toxins do not appear to spread systemically, there is evidence that humoral antibodies against C. difficile Toxin A and B are protective.
In addition to exposure to spores in the healthcare environment or on the hands of caregivers, recent evidence implicates food products (beef, pork, fowl) as a source of C. difficile spores. This food reservoir may be the basis of community-acquired CDI (CA-CDI) as it is now recognized that up to 30% of all CDIs are acquired outside of healthcare facilities and, as discussed by Humphries et al. [2], patients with CA-CDI are more likely to have mild disease, shorter hospital stay and lower rates of mortality. Unlike other enteric vegetative bacterial pathogens in food products that are killed by adequate cooking, the spore form of C. difficile is not killed by cooking [3]. Consumption of C. difficile spores via food or iatrogenic exposure does not automatically lead to disease. Indeed up to 10–20% of healthy people and up to 70% of healthy neonates may harbour this toxigenic C. difficile in their gut but be asymptomatic [3, 4]. It is unknown if this represents transient passage of the ingested spores in the gut where the microbiome keeps C. difficile spores from germinating and replicating thereby preventing toxin production, or whether there can be asymptomatic colonization by toxigenic C. difficile at such low levels that there is no mucosal damage or diarrhoea. Guerrero et al. [5] reported that 12% of asymptomatic patients screened carried toxigenic C. difficile. Although the skin levels and environmental shedding from asymptomatic carriers was lower than from patients with CDI, it has been suggested [5] that asymptomatic carriers may still represent a significant reservoir for transmission within healthcare facilities.
The unique characteristics of C. difficile that include spore formation, asymptomatic carriage and ‘two-hit’ pathogenesis present challenges in terms of optimizing and interpreting diagnostic tests.
Diagnostic testing for toxigenic C. difficile
Over the past 20 years there has been a dramatic revolution in the approach to diagnostic testing for toxigenic C. difficile. Initially in the 1970s the diagnosis of C. difficile-associated diarrhoea was made by culture and subsequent testing of C. difficile isolates to determine if they were toxigenic or not [4, 6]. This was replaced by the cytopathic effect (CPE) assay in the late 1970s and early 1980s that detected biologically active Toxin B directly from the stool sample. Some still consider the CPE assay to be the most clinically relevant diagnostic test as it demonstrates there is sufficient biologically active toxin in stool to cause mucosal damage and diarrhoea. Because culture and CPE assays were labour intensive, costly, time consuming and required specialized expertise, antigen detection assays became the diagnostic test of choice early in the 1990s [4]. However, recent studies have documented that enzyme immunoassay (EIA) for Toxin A and B alone is insensitive and should not be used as a sole diagnostic test for CDI [2, 4, 6–10]. Some researchers advocate that toxigenic culture is the most sensitive diagnostic test [9]. Isolates must subsequently be tested to confirm they are toxigenic. Because toxigenic culture is too slow for clinical testing, multi-step algorithms using glutamate dehydrogenase (GD) antigen as a screen followed by CPE or nucleic acid amplification tests (NAATs) (Table 1) have been recommended [4, 6]. Within the past 5 years there has been a push towards using NAAT alone as the most rapid and sensitive diagnostic test for toxigenic C. difficile [4, 6, 8].
Longtin et al. [7] have recommended that diagnostic testing for C. difficile should be standardized because reportable rates of CDI are dramatically affected by the diagnostic test method or test algorithm utilized. They undertook a one year prospective study and reported that using NAAT alone instead of a multi-step algorithm based on GD antigen, Toxin A/B antigen and CPE assay resulted in a greater than 50% increase in CDI rate in their facility (8.9 cases by NAAT versus 5.8 cases by multi-step algorithm per 10,000 patient days). Their study was the first to report that for patients who were test positive by NAAT alone there was a 3% complication rate compared to the 39% complication rate for patients who were positive by both NAAT and their multi-step algorithm. The lack of standardization in diagnostic testing means the incidence rates reported will vary depending on the test method(s) used. The resultant increase in CDI incidence using NAAT tests compared to other testing algorithms has implications including; Medicare reimbursement penalties in the USA, financial penalties for increased CDI rates in England, target rates in Quebec, Canada.
Additional research is needed to clarify the clinical significance of NAAT positive tests when CPE and antigen tests are negative. As suggested by a variety of published reports [6–8, 10, 11], it may be wrong to assume that higher sensitivity makes for a better C. difficile diagnostic test. Leslie et al. [12] reported that quantitation of C. difficile copy number is reliable and they suggested this added information may help determine when therapy is warranted for NAAT positive tests. They reported that 30.6% of stools that were only positive by NAAT, and had no toxin detected by CPE or antigen testing had low C. difficile copy number/ml. Their data suggests that a large portion of NAAT positive samples fall into this category of ‘questionable’ clinical significance. Vancomycin treatment of asymptomatic C. difficile carriers has been shown to itself stimulate CDI and indeed the authors warned against antibiotic treatment for asymptomatic C. difficile carriers. It may be that detection by NAAT of low organism load represents spores (i.e. no toxin present) or may represent vegetative levels that do not require antibiotic therapy. Dionne et al. [10] reported good correlation between low levels of viable C. difficile and test positivity by NAAT only (i.e. negative by CPE and antigen detection). Furthermore, they were able to demonstrate a correlation between PCR cycle time (CT) and the level of viable C. difficile in the stool sample (Table 2).
Although many published manuscripts and reviews list sensitivity and specificity values, these are all dependent upon what is used as the ‘gold standard’. For C. difficile this is not a simplistic issue. It is clear that detection of toxigenic C. difficile by culture does not always indicate clinically significant disease.
Conclusions
As summarized in Table 3, there are a number of unresolved issues relating to diagnostic testing for C. difficile. For asymptomatic carriers of C. difficile who do not have diarrhoea, the concept of NAAT admission screening and contact precautions for those who test positive has yet to be determined to be beneficial in preventing the spread of CDI. For those patients with diarrhoea it is apparent that CDI rates will vary dramatically depending on the testing algorithm used. It is clear that NAAT used alone as a sole diagnostic test will overestimate CDI rates and could lead to unnecessary antibiotic therapy. The impact is substantive as about one-third of all NAAT positive results fall into this ‘grey area’ of doubtful clinical relevance (i.e. have no detectable toxin in the stool sample and/or are toxigenic culture negative).
In conclusion, a combination NAAT test that provides a quantitative assessment of the load of C. difficile in stool along with detection of C. difficile toxin genes appears to be the ideal combination of data in order to reliably determine which patients have clinically significant CDI and require treatment. However, prospective studies that assess clinical outcome based on this quantitative NAAT testing are needed to confirm that this diagnostic approach is optimal.
References
1. Bartlett JG, Chang TW, Gurwith M, Gorbach SL, Onderdonk AB. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978; 298(10): 531–534.
2. Humphries RM, Uslan DZ, Rubin Z. Performance of Clostridium difficile toxin enzyme immunoassay and nucleic acid amplification tests stratified by patient disease severity. J Clin Microbiol. 2013; 51(3): 869–873.
3. Gupta A, Khanna S. Community-acquired infection: an increasing public health threat. Infect Drug Resist. 2014; 7: 63–72.
4. Tenover FC, Baron EJ, Peterson LR, Persing DH. Laboratory diagnosis of Clostridium difficile infection can molecular amplification methods move us out of uncertainty? J Mol Diagn. 2011; 13(6): 573–582.
5. Guerrero DM, Becker JC, Eckstein EC, Kundrapu S, Deshpande A, Sethi AK, et al. Asymptomatic carriage of toxigenic Clostridium difficile by hospitalized patients. J Hosp Infect. 2013; 85(2): 155–158.
6. Dubberke ER, Han Z, Bobo L, Hink T, Lawrence B, Copper S, et al. Impact of clinical symptoms on interpretation of diagnostic assays for Clostridium difficile infections. J Clin Microbiol. 2011; 49(8): 2887–2893.
7. Longtin Y, Trottier S, Brochu G, Paquet-Bolduc B, Garenc C, Loungnarath V, et al. Impact of the type of diagnostic assay on Clostridium difficile infection and complication rates in a mandatory reporting program. Clin Infect Dis. 2013; 56(1): 67–73.
8. Brecher SM, Novak-Weekley SM, Nagy E. Laboratory diagnosis of Clostridium difficile infections: there is light at the end of the colon. Clin Infect Dis. 2013; 57(8): 1175–1181.
9. Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010; 31(5): 431–455.
10. Dionne LL, Raymond F, Corbeil J, Longtin J, Gervais P, Longtin Y. Correlation between Clostridium difficile bacterial load, commercial real-time PCR cycle thresholds, and results of diagnostic tests based on enzyme immunoassay and cell culture cytotoxicity assay. J Clin Microbiol. 2013; 51(11): 3624–3630.
11. Su WY, Mercer J, Van Hal SJ, Maley M. Clostridium difficile testing: have we got it right? J Clin Microbiol. 2013; 51(1): 377–378.
12. Leslie JL, Cohen SH, Solnick JV, Polage CR. Role of fecal Clostridium difficile load in discrepancies between toxin tests and PCR: is quantitation the next step in C. difficile testing? Eur J Clin Microbiol Infect Dis. 2012; 31(12): 3295–3299.
The author
Michelle J. Alfa, PhD
Boniface Research Centre, Dept. of Medical Microbiology, University of Manitoba, Winnipeg, Manitoba, Canada
E-mail: malfa@dsmanitboa.ca