Diagnostically challenging cases: distinguishing primary from secondary ovarian tumours
Tumours found in the ovaries can be either from primary ovarian tumour processes or metastases (secondary tumours) foremost from colorectal cancer (CRC), appendiceal tumours or stomach cancer. Correctly distinguishing between these tumour subsets using hematoxylin-eosin staining in combination with immunohistochemistry can be problematic [1–3], but is crucial for correct treatment choice. Mutation profiles, generated in a fast and cost-effective way by (targeted) Next Generation Sequencing (NGS), can assist in correctly diagnosing ovarian tumours.
by Stijn Crobach and Prof. Hans Morreau
Background
The ovaries are a preferential location for metastases from, among others, colon, stomach, appendiceal, breast and endometrium carcinomas. The percentage of secondary ovarian tumours (metastases), varies in several reports ranging from 8–30% [4, 5]. Several reasons can be given to explain why the range of percentages is so broad. First, studies are different by design. Some studies are based on autopsy findings, others on prophylactic oophorectomies. Second, differences in incidence of primary tumours can cause a variance in patterns of metastases. For example, stomach cancer has a higher incidence in Japan than in many other countries; therefore, metastases of stomach cancer to the ovaries are expected to be more common in Japan. In general, however, the gastrointestinal tract (GIT) seems to be the main source of ovarian metastases [5].
Macroscopic and histologic approaches
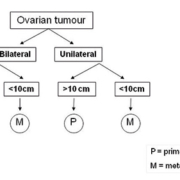
A gross distinction between primary and secondary ovarian tumours can be made taking tumour size and unilaterality versus bilaterality into account [6]. Following the decision tree depicted in Figure 1, it is possible to estimate whether an ovarian tumour is a primary tumour or a metastasis. A unilateral ovarian tumour with a diameter larger than 10 cm is probably a primary tumour. All bilateral and unilateral tumours smaller than 10 cm are much more likely to be metastases.
The histologic characteristics of metastatic GIT ovarian tumours can resemble primary endometrioid and mucinous ovarian tumours, but not serous papillary or clear cell tumours. Thus, based on histology a subset of primary ovarian tumours does not cause diagnostic doubt about the origin of the malignancy. Furthermore, other histologic findings can assist in defining the malignancy. For example, on the one hand, surface involvement by malignant epithelial cells is much more often seen in metastases than in primary ovarian tumours. On the other hand, however, an expansile growth pattern is more often seen in primary ovarian tumours. So, with the help of histopathological findings the characterization of a primary origin or a metastatic process becomes more achievable.
Immunohistochemical approaches
The logical next step in differentiating primary ovarian tumours from metastases is with the use of immunohistochemistry. For example, primary ovarian tumours are classically positive for keratin 7 and negative for keratin 20, whereas colorectal tumours show the opposed staining pattern (keratin 7 negative, keratin 20 positive) [7]. Other markers can also be used, not only to rule out an ovarian origin of the tumour but also to get an idea about the location of the primary tumour. Positivity of intestinal markers [such as carcinoembryonic antigen (CEA) and caudal type homeobox 2 (CDX-2)] can be an argument for an intestinal origin of the tumour cells [8].
Furthermore, when a colon carcinoma is already diagnosed before the ovarian tumour is discovered, the staining profile of the metastasis can be compared with the primary tumour. However, in up to 38% of cases the detection of ovarian metastases precedes the detection of the primary tumours. Also, secondary primary ovarian tumours can occur in patients that anamnestically suffered from another malignancy, complicating the diagnostic procedures. In practice, immunohistochemistry is frequently not fully discriminating. As mentioned, primary ovarian tumours tend to have a Ker7+/Ker20− immunoprofile and colonic metastases a Ker7−/Ker20+ immunoprofile. Nevertheless, keratin 7 positivity can be seen in proximal located GIT tumours, and keratin 20 positivity can also be seen in primary ovarian malignancies. In Figure 2, a guided immunohistochemical decision scheme is shown for complex cases.
Molecular diagnostic approaches
With the combined use of clinical information, histologic features and immunohistochemical staining patterns, differentiating primary tumours from metastases is possible in a substantial subset of cases. With a history of a colorectal tumour and the presentation of a large ovarian mass a few years later showing a similar immunoprofile, it is not difficult to decide that this tumour is a metastasis. Nevertheless, there are cases that are not as clear-cut. In those cases tumour size, unilaterality versus bilaterality and the histologic findings are not discriminating enough to solve the challenge. New approaches using massive parallel DNA sequencing (Next Generation Sequencing; NGS) have emerged in recent years.
Cancer driver genes (oncogenes and tumour suppressor genes) can be screened for DNA mutations in different tumour types. In the Catalogue Of Somatic Mutations In Cancer (COSMIC; http://cancer.sanger.ac.uk/cosmic), literature on these profiles has been compiled [9]. It was hoped that comparing mutational profiles of primary ovarian tumours versus metastases from different organs would reveal specific mutation patterns and/or mutation types in different tumour types.
NGS enables the screening of a large number of genes in a fast and cost-effective way. Previously, Sanger DNA sequencing was used to detect mutations in clinically relevant genes. However, screening complete genes and multiple genes in this way is a time-consuming process. Now, with the introduction of the disruptive NGS technology, it is possible to sequence multiple genes at the same time. NGS will become a standard technique in diagnostics for identifying gene mutations, chromosomal rearrangements and RNA expression/mRNA patterns [10]. One would expect that large scale screening of molecular alterations will results in very specific profiles per tumour type. Each tumour type could be defined by subsets of mutated genes. However, recent studies show that the mutation profiles do not differ so much between tumour types [11]. A few well-known so-called cancer driver genes seem to be important in many malignancies. Other (passenger) mutations, which are also needed in tumorigenesis, seem to be interchangeable. Apparently, there is wide overlap in mutation profiles. Looking at mutations described in the COSMIC database or The Cancer Genome Atlas (TCGA) at the current time, similar mutations can be seen in both primary ovarian tumours and metastases, although with different frequencies. The latter would suggest that the applicability of such tests is limited. However, a more select approach shows that certain genes can be discriminatory.
For example, CTNNB1 mutations are found in primary endometrioid carcinoma of the ovary. CTNNB1 mutations are also found in colon tumours, but only in mismatch repair deficient colon tumours, that do not tend to metastasize to the ovary. This reasoning could also be followed for APC, which is frequently mutated in colon carcinomas but not typically in mucinous and endometrioid primary ovarian carcinomas. However, genes such as these, which show such a ‘black-and-white’ phenomenon, are sparse. Therefore, mutation profiles that are used to guide clinical decision taking will probably be based on combining information from multiple genes. Most of these genes will not provide significant differences on their own, but a combination of odds-ratios will make one diagnosis more probable than the other.
Along with solutions at a mutational level, characterizing the transcriptome, methylation patterns and copy numbers of a tumour could also provide useful information. This field of ‘omics’ has developed rapidly in recent years. In diagnostically challenging cases from unknown primary tumours (UPT) or alternatively named carcinoma of unknown primary (CUP), expression array based assays were developed in order to identify the primary tumours. Genomics will also probably become effective in determining the origin of the tumour. Furthermore, in depth comparison of molecular features of synchronously presenting tumours at different sites might reveal whether the tumours have arisen independently or are clonally related. The readout of these tests can be seen in the context of increased odds-ratios. The use of such tests is still in a premature phase, and not used routinely in clinical practice.
Summary
In conclusion, a combination of the various molecular features will hopefully reveal specific molecular profiles that can be used to correctly identify the origin of malignancies in problematic cases. These techniques are applicable on ovarian tumours, to determine whether tumours are primary ovarian in origin or metastases to the ovaries [12].
References
1. Prat J. Ovarian carcinomas, including secondary tumors: diagnostically challenging areas. Mod Pathol. 2005; 18(Suppl 2): S99–111.
2. Young RH. From Krukenberg to today: the ever present problems posed by metastatic tumors in the ovary. Part II. Adv Anat Pathol. 2007; 14: 149–177.
3. Leen SL, Singh N. Pathology of primary and metastatic mucinous ovarian neoplasms. J Clin Pathol. 2012; 65: 591–595.
4. Moore RG, Chung M, Granai CO, Gajewski W, Steinhoff MM. Incidence of metastasis to the ovaries from nongenital tract primary tumors. Gynecol Oncol. 2004; 93: 87–91.
5. de Waal YR, Thomas CM, Oei AL, Sweep FC, Massuger LF. Secondary ovarian malignancies: frequency, origin, and characteristics. Int J Gynecol Cancer 2009; 19: 1160–1165.
6. Yemelyanova AV, Vang R, Judson K, Wu LS, Ronnett BM. Distinction of primary and metastatic mucinous tumors involving the ovary: analysis of size and laterality data by primary site with reevaluation of an algorithm for tumor classification. Am J Surg Pathol. 2008; 32: 128–138.
7. Ji H, Isacson C, Seidman JD, Kurman RJ, Ronnett BM. Cytokeratins 7 and 20, Dpc4, and MUC5AC in the distinction of metastatic mucinous carcinomas in the ovary from primary ovarian mucinous tumors: Dpc4 assists in identifying metastatic pancreatic carcinomas. Int J Gynecol Pathol. 2002; 21: 391–400.
8. Groisman GM, Meir A, Sabo E. The value of Cdx2 immunostaining in differentiating primary ovarian carcinomas from colonic carcinomas metastatic to the ovaries. Int J Gynecol Pathol. 2004; 23: 52–57.
9. Bamford S, Dawson E, Forbes S, Clements J, Pettett R, Dogan A, Flanagan A, Teague J, Futreal PA, Stratton MR, Wooster R. The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br J Cancer 2004; 91: 355–358.
10. Natrajan R, Reis-Filho JS. Next-generation sequencing applied to molecular diagnostics. Expert Rev Mol Diagn. 2011; 11: 425–444.
11. Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr, Kinzler KW. Cancer genome landscapes. Science 2013; 339: 1546–1558.
12. Crobach S, Ruano D, van Eijk R, Fleuren GJ, Minderhout I, Snowdowne R, Tops C, van Wezel T, Morreau H. Target-enriched next-generation sequencing reveals differences between primary and secondary ovarian tumors in formalin-fixed, paraffin-embedded tissue. J Mol Diagn 2015; 17: 193–200.
The authors
Stijn Crobach BSc; Hans Morreau MD, PhD
Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands
*Corresponding author
E-mail: j.morreau@lumc.nl