DNA microarrays for SNP profiling in thrombosis and hemochromatosis
Specialized diagnostic DNA microarrays provide fast and reliable determination of factor V and factor II gene mutations associated with thrombosis or HFE gene defects linked to hereditary hemochromatosis. The simple microarray procedure includes fully automated data analysis and can be performed on whole blood samples, circumventing the need for preanalytical DNA isolation. Patient genotyping aids diagnosis in symptomatic individuals and risk assessment in healthy individuals, thus facilitating decision making in therapy and prevention.
by Dr Jacqueline Gosink
Laboratory analysis of genetic determinants is gaining momentum as ever increasing numbers of disease-associated alleles are discovered. With cutting edge diagnostic microarray technology, newly identified DNA parameters can progress rapidly from the research laboratory to routine diagnostics. Microarray platforms such as the EUROArray provide quick and easy determination of DNA mutations, enriching diagnosis and risk evaluation in a range of genetically linked diseases.
This article focuses on DNA microarray systems for genetic analysis in two common hereditary hematological disorders. The first detects single nucleotide polymorphisms (SNPs) in the factor V (FV) and/or factor II (FII) genes that lead to thrombosis and embolism. The second identifies up to four SNPs in the HFE (high iron) gene that contribute to hereditary hemochromatosis.
Thrombosis and embolism
Deep and superficial venous thrombosis and thromboembolism of the brain, lung and coronary vessels are among the most frequent causes of death, especially in western industrialized countries. These conditions result from a combination of genetic susceptibility and exogenous factors such as old age, immobility, smoking, diabetes mellitus, pregnancy, oral contraceptives or hormone replacement therapy. Notably, more than half of all cases can be attributed to genetic factors, particularly if the disease occurs before the age of 45 without any obvious external factors or at an atypical location.
The most important and most frequent genetic risk factors are the FV Leiden 1691G>A mutation (APC resistance) and the FII 20210G>A mutation in the prothrombin gene. These DNA mutations result in amino acid substitutions which disrupt the blood coagulation functions of FV and FII.
Factor V and II mutations
In healthy individuals activated FV is normally prevented from triggering coagulation by proteolytic cleavage catalysed by activated protein C (APC) and its cofactor protein S. Persons with the FV Leiden mutation exhibit an altered form of FV resulting from an exchange of the amino acid at position 506 from arginine to glutamine. The modified structure of FV makes it resistant to inactivation by APC (APC resistance), which leads to hypercoagulability and an increased risk of thrombosis. More than 95% of cases of APC resistance are caused by the autosomal, dominant FV Leiden mutation. In Europe around 3-7% of the population is a heterozygous carrier. In these individuals the thrombosis risk is 3-8 times higher than in non-affected persons, and if oral contraceptives are taken up to 30 times higher. The homozygous FV Leiden mutation occurs in around 0.2% of the European population and is associated with a 50-100-fold increased risk of thrombosis.
The 20210G>A mutation in the FII gene leads to a raised plasma concentration of the coagulation factor prothrombin via an as yet unidentified mechanism. The resulting thrombosis can be venous or arterial. The heterozygous genotype is present in 1-3% of the population in Europe and is associated with a 3-fold higher risk of deep venous thrombosis. If oral contraceptives are taken, the risk of venous thrombosis is increased 16-fold and of brain venous thrombosis up to 150-fold.
The factor V and factor II gene defects have an additive effect, and thrombophilia patients who exhibit the FII 20210G>A mutation often also have the FV Leiden mutation. In these patients the risk of venous thrombosis is elevated by a factor of 20.
Genetic analysis of FV and FII mutations is of outstanding importance in individuals with a high thrombosis risk based on their personal or family history, as well as in patients with unexplained recurrent miscarriages, biochemically proven resistance to APC or proven protein C or protein S deficiency. Genetic risk determination should also be undertaken before prescribing oral contraceptives or hormone replacement therapy to women with a familial tendency to thrombosis, especially young smokers.
Hereditary hemochromatosis
Hereditary hemochromatosis is the most frequent autosomal, recessive inherited metabolic disorder and is characterized by increased resorption of iron in the upper small intestine. The augmented iron uptake leads to an increase in the total iron content in the body from around 2–6 g (normal value) to up to 80 g. Since the human body cannot excrete the excess iron, it is deposited in various organs such as the liver, pancreas, spleen, thyroid gland, pituitary gland, heart and joints. In untreated patients irreversible damage occurs, resulting in an increased risk of cardiomyopathy, arthropathy, diabetes mellitus, liver cirrhosis and liver and pancreas carcinoma. Most cases of hereditary hemochromatosis are caused by defects in the HFE gene, which lead to functional flaws in the encoded iron regulatory protein.
HFE mutations
There are four SNPs in the HFE gene that are associated with hereditary hemochromatosis. The two most frequent, representing 90% of cases, result in the amino acid substitutions C282Y or H63D which cause a loss or reduction of the physiological function of the Hfe protein. The penetrance of the mutations is dependent on age and gender. Thus, the disease does not necessarily manifest itself in all carriers of these mutations. The strongest disease association is observed in patients with a homozygous C282Y mutation, whereby the penetrance is much lower in young women than in men due to menstruation. While 80% of men under 40 with this gene defect develop hemochromatosis, less than 40% of women do so. The penetrance increases to 95% of men and 80% of women for the population group of over 40 year olds. The two further SNPs in the HFE gene that are associated with hereditary hemochromatosis are S65C, which results in an amino acid substitution in the Hfe protein, and E168X, which causes early termination of protein synthesis, whereby both of these mutations are rare.
Around 10% of the population in northern Europe is heterozygous for one of the disease-associated mutations in the HFE gene and 0.3–0.5% is homozygous. New studies show that 90–100% of hemochromatosis patients exhibit homozygous gene defects. However, even a mutation in one HFE allele is sufficient to cause at least minor abnormalities in iron metabolism. The early identification of HFE gene defects enables suitable preventative measures to be implemented, for example a reduction in the consumption of high-iron-containing foods.
Simple microarray analysis
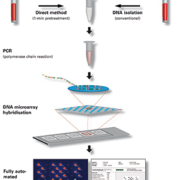
DNA mutations associated with thrombosis and hemochromatosis can be reliably determined using DNA microarray systems such as EUROArray [1, 2]. This microarray system provides fast and efficient SNP detection with fully automated data analysis, and can easily be used by persons unfamiliar with molecular biology. A special feature of the thrombosis and hemochromatosis microarray procedures is the use of pretreated whole blood as sample material, which eliminates the need for a preanalytical DNA isolation step. The hands-on processing time for the direct procedure is thus reduced to as little as 1.5 minutes per sample.
In the microarray procedure [Figure 1], the sections of DNA containing the disease-associated alleles are amplified by multiplex polymerase chain reaction (PCR) using highly specific primers. During this process the PCR products are labelled with a fluorescent dye. The PCR mixture is then incubated with a microarray slide containing immobilized DNA probes [Figure 2]. The PCR products hybridize with their complimentary probes and are subsequently detected via the emission of fluorescence signals. The evaluation of the microarrays [Figure 3] proceeds quickly and objectively using the special microarray scanner and EUROArrayScan software. The software interprets the results, produces patient genotype reports, and archives all data and patient information [Figure 4]. It can be integrated seamlessly into existing laboratory software.
Reliable biochip technology
EUROArrays are based on proven biochip technology which has been adapted for DNA analysis. Each biochip is composed of DNA spots of wild type and mutant alleles and contains in addition integrated control sequences to verify correct performance of the test. The microarray slides are incubated using the established TITERPLANE technique, which provides standardized, parallel incubation of multiple samples. Up to five samples can be analysed per slide. The reproducibility and convenience of the analysis is further enhanced by ready-to-use PCR reagents and meticulously designed amplification primers and hybridisation probes. The entire procedure from sample arrival to report release is IVD validated and CE labelled.
In clinical evaluation using molecular genetically precharacterized samples, each microarray demonstrated a sensitivity of 100% and a specificity of 100% [Table 1]. Homozygous and heterozygous genotypes were reliably discriminated for every position.
Comprehensive microarray range
The thrombosis diagnostic microarray system is available in different constellations for separate or parallel analysis of the FV Leiden and FII 20210G>A mutations, while the hemochromatosis microarray system is available in two versions encompassing either just the two most frequent mutations C282Y and H63D or, for a more extensive analysis, the four disease-associated mutations C282Y, H63D, S65C and E168X.
In addition to the determination of FV/FII and HFE mutations, EUROArray technology can also be used to analyse further genetic risk factors such as HLA-DQ2/ DQ8 in celiac disease, HLA-Cw6 in psoriasis or HLA-B27 in ankylosing spondylitis. New parameters soon to be added to the platform include HLA-DR Shared Epitope in the diagnosis of rheumatoid arthritis and human papilloma virus detection and subtyping.
Summary
The current pace of genetic discoveries combined with advances in microarray technology is resulting in a plethora of novel DNA tests for the routine diagnostic laboratory. New DNA microarrays for rapid identification of thrombosis-associated mutations in the factor V/factor II genes and hemochromatosis-linked mutations in the HFE gene have greatly enhanced diagnosis and risk evaluation in susceptible individuals. Early awareness of a genetic predisposition enables individuals to adopt appropriate lifestyle or medical interventions to reduce the impact or even prevent development of these debilitating diseases.
References
1. Voss J. et al. to be presented at IFCC EuroMedLab, Milano, Italy (2013).
2. Axel K. et al. to be presented at IFCC EuroMedLab, Milano, Italy (2013).
The author
Jacqueline Gosink PhD
Euroimmun AG
Luebeck, Germany