Evaluation of a highly automated fecal calprotectin assay for the differential diagnosis of IBD or IBS
Fecal calprotectin is an effective biomarker in the differential diagnosis of inflammatory bowel disease (IBD) or irritable bowel syndrome (IBS). Since the National Institute for Health and Care Excellence (NICE) recommended its use there has been a significant increase in demand for analysis. New methods on mainline chemistry analysers can be implemented in response to the increase in workload.
by Sally Willett, Pamela Bowe, Frankie Leslie and Wayne Bradbury
Introduction
Chronic abdominal pain with diarrhea or constipation are common presenting symptoms in general practice. The differential diagnosis in this patient population is varied, but includes irritable bowel syndrome (IBS) or inflammatory bowel disease (IBD).
IBS is a chronic, relapsing and often lifelong disorder associated with disordered defecation and abdominal distention. It is not associated with any distinctive pathology and although it is troublesome for the patient it is not associated with any serious comorbidity. IBS is a relatively common diagnosis with a prevalence of 10–20% in the general population [1].
IBD is a much more serious condition, associated with a high morbidity. The term IBD includes Crohn’s disease and ulcerative colitis, conditions in which gastrointestinal inflammation can lead to major complications. Patients may require surgery and are at increased risk of colorectal cancer. Evolving treatment options, including novel drugs and surgery, aim to secure and maintain remission [2].
It is important to distinguish IBD from non-IBD, such as IBS, so that conditions can be appropriately managed and monitored. Endoscopy with histological examination of biopsy samples remains the gold standard in differentiating IBD and IBS, but is very expensive, time consuming and invasive. Conventional diagnostic testing included markers of inflammation including C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). However, these markers cannot localize inflammation to the gut. There has been intensive research into fecal biomarkers, specific for gastrointestinal inflammation over the last decade.
Calprotectin is a small calcium binding protein which contributes ~60% of the protein content of the cytosol in neutrophils [3]. During the intestinal inflammation observed in patients with IBD neutrophils migrate to the intestinal mucosa. As the inflammatory process damages the mucosal architecture the neutrophils are shed into the lumen and calprotectin is detectable in the feces. A raised fecal calprotectin concentration (>50 µg/g) has been shown to have a good diagnostic sensitivity and specificity for the detection of IBD [4].
Analytical methods for the detection of calprotectin in feces have evolved since the original enzyme-linked immunosorbent assay (ELISA) method was described in 1992 [5]. Commercial immunoassays are now available and quantitative lateral flow immunochomatographic point-of-care tests have been marketed to generate rapid results in the clinic setting. Many laboratories still use ELISA technology to analyse fecal samples for calprotectin. Such analysis is relatively labour intensive and often fecal extracts are run in duplicate at increased cost.
Since the National Institute for Health and Care Excellence (NICE) recommended the use of fecal calprotectin in primary care [2], there has been a significant increase in demand for this test. We investigated the performance of the new BÜHLMANN fCALTM turbo method which is CE marked for use on a number of mainline chemistry analysers. Implementation of this method has the potential to streamline analysis, relieving staff time and reducing cost.
Method
The BÜHLMANN fCALTM turbo particle enhanced turbidimetric immunoassay (PETIA) method on the Roche Cobas 6000 (c501) was compared to the BÜHLMANN Calprotectin ELISA method on the Dynex DS2. The study was performed within the Blood Sciences Department at North Cumbria University Hospitals.
The PETIA method uses polystyrene nanoparticles coated with specific antibodies to bind calprotectin in fecal extracts. Calprotectin in the sample mediates immune-particle agglutination and the resultant increase in turbidity is quantified by optical density.
Fecal samples were extracted using the BÜHLMANN CALEX® extraction device prior to analysis on both methods. Fifty-eight patient samples were analysed and results compared using regression analysis. Intra-assay precision was determined using 10 replicates of patient samples and inter-assay precision was calculated using 17 replicates of internal quality control material. NEQAS samples were analysed and bias relative to the all laboratory trimmed mean (ALTM) was assessed.
Results and discussion
Comparison of patient results showed good correlation (R2=0.97) consistent with previous studies [6, 7]. Regression analysis produced the following equation:
fCALTM turbo = (1.14×DS2 result)−23.42
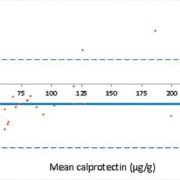
The fCALTM turbo method demonstrated a negative bias at concentrations <100 µg/g and a positive bias at higher concentrations when compared with the ELISA method (Fig. 1), which has also been observed by De Sloovere et al. [6]. The positive bias observed at higher concentrations is accounted for in local guidelines. Since the initial evaluation a field safety notice (FSN) was distributed informing users that a positive bias of 15.6% was observed using the BÜHLMANN CALEX® extraction devices. This has subsequently been corrected with the CALEX® Cap “N” devices. After the introduction of the revised extraction devices external quality assurance (EQA) results have improved, and local results show a mean bias of 54 µg/g from the NEQAS ALTM (Fig. 2). A commutable reference material for calprotectin is required to define analytical accuracy in the future.
Intra-assay precision, as determined by percent coefficient of variation (%CV), was 3.1% and 1.3% at concentrations of 48 µg/g and 247 µg/g respectively (n=10). Inter-assay precision was 3.3% at 73 µg/g and 1.1% at 247 µg/g (n=17). This is consistent with De Sloovere et al. who demonstrated %CVs of ~3% using the fCAL turbo method [6]. Since running the method routinely the internal quality control data shows a running %CV of 4.5% at 75 µg/g and 2.6% at 245 µg/g (n=23).
Historically, fecal samples required weighing and diluting in extraction buffer before analysis, which was very labour intensive and prone to error. The introduction of extraction devices has simplified the pre-analytical steps significantly. The introduction of the PETIA method into our laboratory has further simplified analysis and reduced staff time, as the fecal extracts are loaded directly onto the Cobas 6000 in barcoded CALEX tubes. The PETIA method has a large analytical range (20–1800 µg/g feces) reducing the requirement for costly repeat analysis on dilution. Although the ELISA method favours batch analysis, the PETIA method is suitable for random access testing, improving assay turnaround times. An additional wash step is implemented to eliminate carry over between fecal and blood samples.
Conclusion
It is important to accurately differentiate IBD from IBS so that appropriate patient care pathways can be instigated. The methodologies available for the quantification of fecal calprotectin have evolved significantly over the last decade. The BÜHLMANN fCALTM turbo PETIA method on the Roche Cobas 6000 (c501) demonstrated acceptable performance and is suitable for routine use within a diagnostic laboratory.
References
1. National Institute for Health and Clinical Excellence (NICE). Irritable bowel syndrome in adults: diagnosis and management. NICE clinical guideline 61, 2008.
2. NICE. Faecal calprotectin diagnostic tests for inflammatory diseases of the bowel. NICE diagnostic guideline 11, 2013.
3. Fagerhol M, Dale I, Andersson T. A radioimmunoassay for a granulocyte protein as a marker in studies on the turnover of such cells. Bull Eur Physiopathol Respir 1980; 16(Suppl): 273–282.
4. Walsham N and Sherwood R. Fecal calprotectin in inflammatory bowel disease. Clin Exp Gastroenterol 2016; 9: 21–29.
5. Roseth AG, Fagerhol MK, Aadland E, Schiønsby H. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol 1992; 27: 793–798.
6. De Sloovere M, De Smet D, Baert F, Debrabandere J, Vanpoucke HJM. Analytical and diagnostic performance of two automated fecal calprotectin immunoassays for detection of IBD. Clin Chem Lab Med 2017; 28: 1435–1446.
7. Nilsen T, Sunde K, Hansson L, Havelka AM, Larsson A. A novel turbidimetric immunoassay for fecal calprotectin optimized for routine chemistry analysers. J Clin Lab Anal Analysis 2017; 31: 1–6.
The authors
Sally Willett FRCPath, Pamela Bowe* MSc, Frankie Leslie BSc, Wayne Bradbury FRCPath
Blood Sciences, North Cumbria University Hospitals NHS Trust, Cumberland Infirmary, Carlisle, UK
*Corresponding author
E-mail: Pamela.Bowe@ncuh.nhs.uk