Background
Sample preparation is important in any type of chromatography analysis. While it can add on extra time, the process of cleaning up samples before injection onto a system results in a range of benefits to the analyst – better recoveries, more reproducible analysis, less downtime of instruments, reduction of troubleshooting, as well as less complex chromatograms due to the reduction of unwanted compounds being injected. All of these can result in time saved which could be needed for repeated work or maintenance on instruments.
Traditional SPE products consist of a loose-filled resin sandwiched between two frits. While this is known to work, it can come with some problems which can complicate analysis or result in poor data being produced. These problems are a result from how the product is packed into a well or cartridge – voiding can occur under the top frit, channels could form through the resin bed which can cause less efficient interactions between the resin and the analyte(s) or there could be variation on compression or resin weight that was dosed into each product.
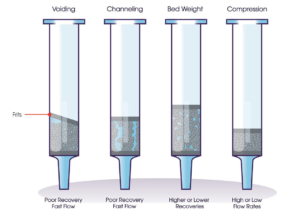
Figure 1. Common issues associated with loose-filled SPE methods
The Microlute® CP SPE products consist of a unique hybrid design of a solid interconnected network of evenly distributed pores combined with retentive media. The advantage of this design is that flow through the product is consistent and increases the interaction between the analytes and retentive media present within the structure. These two features combined results in a product which offers both high recovery values as well as reproducible results. This technical note uses the 30 mg Microlute® CP Strong Cation Exchange (SCX) 96 well plate to compare performance in recoveries and reproducibility against five competitor loose-filled 30 mg SCX products.
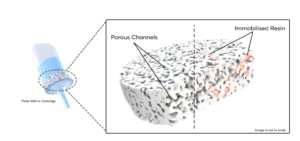
Figure 2. Schematic of the hybrid polymeric structure showing the porous structure of the frit with the active resin immobilised throughout the pore structure
Introduction
The Microlute® CP SCX product is a mixed-mode polymeric SPE product – a combination of ion exchange and reversed phase. This results in a product that has two retention mechanisms which can be fine-tuned to allow more flexibility in the SPE method. The reversed phase functionality allows for separation of analytes on hydrophobic interactions, allowing for retention to be altered by organic modifier concentration. Whereas ion exchange allows for selective strong ionic interactions between the resin and the charged analytes. The introduction of polymeric resins to SPE has resulted in some extra advantages over using silica-based resins [1]. These include:
- Polymeric structures do not contain any of the highly active sites found in These include silanol groups which can cause unwanted secondary interactions with analytes which in some cases could cause irreproducible recoveries.
- Silica’s structure is also very susceptible outside of the pH range of 2 – 5. If pH is outside this range, hydrolysis of the silica or bonded functional group on the surface could occur. [2] This will result in very poor and irreproducible recoveries. On the other hand, polymeric resins are resistant to pH allowing them to work over the whole pH range (pH 0 – 14).
- Functional groups bonded to a silica surface need to be conditioned with an organic solvent to activate the retention mechanism then equilibrated with an aqueous Whilst polymeric resins do not necessarily need to go through this conditioning step.
- Polymeric resins are less sensitive to drying out during the SPE process where silica resin can become dry and lose their retentive function. [3]
Strong ion exchange does not work well for strongly acidic or basic analytes (analytes which have a charge over the whole pH range) due to the irreversible binding of charged analyte to the charged ion exchange resin. Weak analytes do work well with strong ion exchange. This is because it is possible to turn the analyte’s charge on or off for a weakly acidic or basic analyte allowing selective binding with a change of pH. To optimise this binding, the 2 pH rule is applied:
- When the pH of the solution in which an analyte is dissolved is equal to the pKa value of the analyte, the analyte is 50% ionised, pH can then be used to adjust how ionised a compound is:
◊ Acidic: 100% ionised: 2 pH above pKa or 100% un-ionised: 2 pH below pKa
◊ Basic: 100% ionised: 2 pH below pKa or 100% un-ionised: 2 pH above pKa

Figure 3. A diagram to show the 2 pH rule for a weak acid compound and weak basic compound , with a pKa of 4 and 8, respectively
For a typical strong ion exchange method, analytes are pre-treated to a pH where they are charged and then loaded onto the resin. This allows them to bind strongly to the resin with the ionic interactions. The same pH will be maintained on the wash steps of the method allowing the analyte(s) to keep binding to the resin while washing off interfering com- pounds. To allow elution, pH is changed to neutralise the charge allowing the analyte(s) to stop binding to the resin and elute from the product.
Experimentals
Chemicals
Caffeine, salbutamol, procainamide, atenolol, pindolol, propranolol, desipramine, protriptyline, imipramine, amitriptyline, nortriptyline, formic acid, methanol, water, 35% ammonia solution.
Sample Preparation
A stock of 1,000 µg/ml of all the basic analytes was made in methanol. A basic load solution was made by diluting 500 µL of the stock solution to 50 mL with water containing 0.1% (v/v) formic acid.
Solid Phase Extraction Method
For both the Microlute® CP SCX product and competitor products, a total of 12 wells were tested. Each well tested was conditioned with 1,000 µL methanol, then equilibrated with 1,000 µL of water. 1,000 µL of basic load solution was then loaded onto the plate in full. Once loaded, 1,000 µL of water containing 0.1% (v/v) formic acid solution in water was used to wash the sorbent. Followed by a strong organic wash of 0.1% (v/v) formic acid solution in methanol.
To elute the analytes of interest, 500 µL of methanol containing 5% (v/v) ammonia was used. The eluent was then evaporated to dryness at 35°C under N2 using a Porvair Sciences Ultravap® Levante (# 500226). A repeat elution was performed using another 500 µL of methanol containing 5% (v/v) ammonia and evaporated to dryness with the same method.
To reconstitute each sample, to each collection vial, 10 µL of 1,000 µg/ml caffeine (ISTD) was added, followed by 790 µL of 60% (v/v) methanol/water containing 0.1% (v/v) formic acid to create a 12.5 µg/mL solution. A further dilution was made using 40 µL of 12.5 µg/mL solution with 760 uL of 60% (v/v) methanol/water containing 0.1% (v/v) formic acid, creating 0.625 µg/mL solutions ready for injection.
Results and Discussion
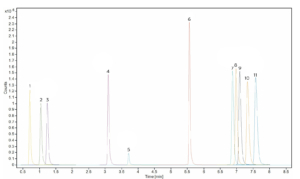
Figure 4. Chromatogram of basic analytes calibration standard.
Peak assignments can be found in Table 3.
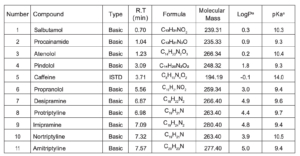
Table 3. Properties and MS parameters for the basic compounds analysed – aPredicted value from Pubchem [4]
Recovery Comparisons against Competitors
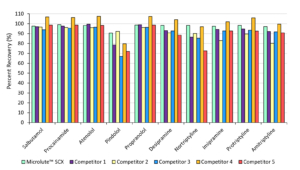
Figure 5. Analyte recovery comparisons against equivalent competitor SPE products.
Reproducibility Comparison against Competitors
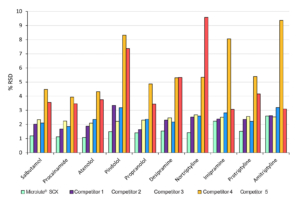
Figure 6. Analyte %RSD comparisons against equivalent competitor SPE products.
For reproducibility, a lower %RSD means the recovery was more reproducible. The analyte’s %RSD values can be seen in Figure 6. The Microlute® CP SCX managed to maintain a %RSD value of less than 2.6% for every compound analysed. It outperformed every competitor for reproducibility on each compound with only amitriptyline being closely matched. There was no issue of irreproducible results for either the most extreme hydrophilic compound or the most hydrophobic. This again shows that the Microlute® CP SCX product is performing excellently across a wide range of basic analytes.
Summary
The Microlute® CP SCX 30 mg 96 well plate can effectively retain a wide range of hydrophilic and hydrophobic basic compounds. It offers advantageous recoveries across the range of different classes of analytes. Lower %RSD values are seen when comparing against competitor plates for every compound analysed in this study – 1.6%RSD on average for the Microlute® CP SCX compared to the 2.4%RSD (best competitor) and 5.9%RSD (worst competitor). This ensures the product gives reliable and reproducible results which is an important metric in testing where confidence in the data output is required.
References
- F. Poole, Solid-Phase Extraction, Elsevier, 2020, p. Chapter 3: Porous polymer sorbents.
- V. Brady and J. V. Walther, “Controls on silicate dissolution rates in neutral and basic pH solutions at 25°C,” Geochimica et Cosmochimica Acta, vol. 53, no. 11, pp. 2823-2830, 1989.
- N. Qureshi, G. Stecher, C. Huck and G. K. Bonn, “Preparation of polymer based sorbents for solid phase extraction of polyphenolic compounds,” Central European Journal of Chemistry, vol. 9, no. 2, pp. 206-212, 2011.
- “PubChem,” National Library of Medicine, [Online]. Available: https://pubchem.ncbi.nlm.nih.gov/ . [Accessed 25 04 2021].
—————————————————————————–
Ordering Information
Find out more about the Microlute® hybrid technology at www.microplates.com/microlute-cp/
Check out the range of Microlute® sample preparation products at www.microplates.com/microlute/
EU/Row Enquiries
Technical Support
Phone
+441978 66 11 44
USA Enquiries
Technical Support
Phone
+1856 696 3605
Copyright 2021. Porvair Sciences Ltd. All rights reserved. Whilst every effort has been made to ensure the accuracy of this document, due to continuous product development, the data contained is subject to constant revision and Porvair Sciences Ltd. reserves the right to change, alter or modify its contents. Porvair Sciences and JG Finneran Associates, Inc., and Kbiosystems are divisions of Porvair plc.