Faster viral load results for an improved clinical service
With 1,300 beds and over 6,000 employees, the Hospital Universitario 12 de Octubre in Madrid is one of the largest hospitals in Spain, serving a population of more than 500,000 people in and around the capital. It is an important teaching and research centre with a number of areas of expertise, including organ transplantation and the diagnosis and treatment of cancer.
The hospital’s Clinical Microbiology Department has a significant serology workload, processing more than 250 serology samples every day. This includes viral load testing for targets such as cytomegalovirus (CMV), hepatitis B virus (HBV), hepatitis C virus (HCV) and human immunodeficiency virus type 1 (HIV-1).
The serology laboratory faces a number of challenges that need to be addressed in order to meet future workload and service user requirements. Not least, the available space in the laboratory is limited due to the instrumentation that is required. Our existing viral load method requires separate sample preparation and amplification/detection platforms, and involves considerable manual intervention. Furthermore, as samples are processed in batches, this requires additional space for pre-analytical sample storage.
On reviewing our processes, we identified the need for increased automation within the laboratory, to reduce the number of manual steps and improve workflow efficiencies, and improve laboratory response times.
Evaluating new technology
In 2014-2015, we had the opportunity to evaluate a new, fully automated, random access platform for viral load analyses. The DxN VERIS Molecular Diagnostics System (Beckman Coulter) consolidates DNA extraction, nucleic acid amplification, quantification and detection onto a single automated instrument for a number of molecular targets. We evaluated the performance of the VERIS assays for CMV, HBV, HCV and HIV-1 using standard and control samples, as well as clinical samples, comparing them to our existing viral load method (COBAS Ampliprep®/COBAS TaqMan® assays, Roche).
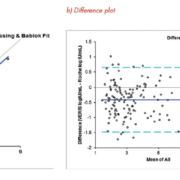
All four assays were found to have comparable performance to our existing method, demonstrating excellent sensitivity, specificity and precision [1,2]. The correlation between both methods for HBV viral load quantification, for example, is shown in figure 1. A precision analysis for the VERIS HBV assay, which was calculated for five levels tested in duplicate over 20 days, gave a ‘within run’ standard deviation of ≤0.09 Log IU/mL and a ‘between run’ standard deviation of ≤0.09 Log IU/mL (table 1). Moreover, repeated analysis of negative samples alongside high positive samples at different rack positions showed no cross contamination, giving confidence in results. This random access technology provided the first result in just 75 minutes for HBV and CMV DNA, and in 90 minutes for HCV and HIV-1 RNA, with subsequent results every 2.5 minutes.
Our experiences in evaluating the DxN VERIS system led us to appreciate its potential as an enabler for an improved molecular biology clinical service. The increased automation and random access offer workflow improvements that simplify laboratory tasks and reduce the potential for human error. Furthermore, its overall performance and ease of use facilitated the smooth introduction of the technology in our laboratory.
Rapid results inform prompt treatment decisions
Early in 2016, we began to use the DxN VERIS System routinely for HBV and HCV viral load quantifications. Our annual volume of HBV and HCV samples is around 7,000 and, as a clinical laboratory working closely alongside medical staff, our viral load results support timely clinical decision making and subsequent patient management. In this respect the DxN VERIS system is ideal for our needs, providing same day results to our outpatient clinics.
One of the most important aspects of the system for our laboratory is the ability to process samples as they are received in the laboratory. With our previous method, we had to work in batches of 24 or 48, collecting and storing samples throughout the day (or overnight) until we had a sufficient number of samples for a single run. Then results were not available until the entire run was completed. This had a huge impact on response times.
Now, with the random access capabilities of the DxN VERIS system, this has changed. We receive samples around the clock and we are able to run them straight away. This has improved our response times significantly, from 24-28 hours to just 4-5 hours from sample receipt, and with comparable quality of results compared to our previous method.
At the moment we enter results into the patient record manually, but soon we will be moving to a barcode system that will transfer all details and results into the electronic patient record automatically, saving time and further reducing opportunities for human error.
Expanding laboratory capabilities
The DxN VERIS system has been well received by laboratory staff and has expanded our service capabilities. Fully automated from the loading of samples to obtaining results, it is easy to operate by laboratory technicians of all abilities. In addition, since it involves minimal manual intervention and fewer steps than our previous method, there is less opportunity for error and staff have more time to perform other important tasks in the laboratory.
One of our objectives as a clinical microbiology department is to offer a more complete panel of assays on a 24-hour basis. Previously, this was not possible for molecular diagnostic investigations such as HBV and HCV viral loads, because it was not practical to run one or two samples at a time on our previous system. The random access and ease of use of the DxN VERIS system has enabled us to operate our HBV/HCV viral load service 24 hours per day, making it ideal to meet the variable needs of our laboratory in terms of workload volume and response times.
For further information about the DxN VERIS Molecular Diagnostic System and the VERIS assays currently available, please contact: Tiffany Page, Senior Pan European Marketing Manager Molecular Diagnostics, Email: info@beckmanmolecular.com or visit www.beckmancoulter.com/moleculardiagnostics
References
1. Rafael Delgado (2015) Evaluation in a Clinical Setting of the General Performance of DxN VERIS CMV and HBV Viral Load Assay. Oral presentation, ECCMID, Copenhagen.
2. Gutiérrez, F, Zurita, S, Pérez-Rivilla, A and Delgado, R (2015) Evaluation of the Automated DxN VERIS System for Human Immunodeficiency Virus Type-1 (HIV-1) and Hepatitis C Virus (HCV) Viral Load (VL) Monitoring. Poster presentation ESCV, Edinburgh.
The author
Rafael Delgado, Head of Clinical Microbiology Hospital
Universitario 12 de Octubre, Madrid, Spain.