Fecal immunochemical testing in colorectal cancer screening
Most of the colorectal cancer (CRC) screening programmes are based on fecal occult blood tests (FOBT). However, there is little information on the utility of FOBT in familial-risk CRC screening. A recently published study has evaluated the diagnostic accuracy of fecal immunochemical tests (FIT) for CRC and advanced colonic neoplasia detection in this population.
by Dr Inés Castro and Dr Joaquín Cubiella
Relevance of colorectal cancer screening
Colorectal cancer (CRC) is the third most common cancer worldwide and the second leading cause of cancer deaths in developed countries [1]. Several factors have been related to the risk of developing CRC. Age, gender and CRC familial history, especially if there are first-degree relatives (FDR) with CRC, are the strongest associated risk factors [2]. Furthermore, CRC risk is directly related to the number of FDRs and inversely related to the age of youngest FDR [3, 4].
Evidence from several studies has shown that CRC screening is effective for CRC prevention in the average-risk population (asymptomatic individuals between 50 and 69 years) [2]. Indeed, both flexible sigmoidoscopy and fecal occult blood tests (FOBT) have been shown to reduce CRC-specific mortality and incidence in randomized controlled trials [2, 5, 6]. This reduction is based on CRC early detection and prevention through adenoma detection and endoscopic resection [2]. Accordingly, these two strategies, along with colonoscopy, have been universally accepted and recommended for CRC screening [2]. In Europe, annual or biennial FOBT is the most widespread CRC screening strategy.
Approximately 95% of CRCs develop on advanced adenomatous polyps (larger than 10 mm, with high-grade dysplasia or villous architecture). These lesions, and early CRC, are characterized by intermittent microscopic blood loss in the stool that can be detected with FOBTs before they are clinically apparent.
Advantages and disadvantages of the different FOBTs
FOBTs detect through blood or blood products (such as globin) in feces with different methods. Mainly, there are two types of FOBTs: chemical (cFOBT) or immunological (FIT).
Chemical fecal occult blood tests
cFOBTs are simple, qualitative tests that use different indicators such as guaiac resin (Hemoccults, Hemoccult II®, SmithKline Diagnostics, Sunnyvale, California, USA), orthotolidine (Hematest®, MilesLaboratories, Elkhart, Indiana, USA) or benzidine (Hemofec®, Med-Kjemi AS, Asker, Norway). By an oxidative reaction and in the presence of a developer solution, hemoglobin seudoperoxidase activity produces a colour change in the paper impregnated with guaiac resin, orthotolidine or benzidine.
However these tests have multiple drawbacks: first, they are not specific to human hemoglobin. Oxygenation reactions may occur with foods with peroxidase activity such as vegetables or uncooked red meat, so it is recommended to withdraw them from the diet for 3 days before sample collection to reduce false positive results. In addition, hemoglobin from the upper gastrointestinal digestive tract is also detected. So, gastrolesive drugs (NSAIDs or aspirin) should be interrupted 7 days before testing. Besides, two stool samples on three consecutive bowel movements are required, limiting adherence to screening programmes [7]. Furthermore, as it is a qualitative test, its result is subjected to interpretation. Finally, their sensitivity for CRC and advanced adenoma detection is low. This is associated with an interval CRC rate ranging between 30 and 50%.
Fecal immunochemical tests
FITs are based on the reaction of monoclonal or polyclonal antibodies specific for human hemoglobin, albumin or other fecal blood components. Thus, they require no dietary or pharmacological restriction, as long as they do not react with blood from upper digestive tract or with any food component. The greatest advantage is determined by its ability to detect and quantify fecal hemoglobin concentrations 7 to 15 times lower than those detected by chemical tests, significantly improving CRC and advanced adenoma detection sensitivity. Besides, FITs allows a reliable and accurate automated analysis, avoiding subjective interpretation. Up to 50 samples an hour can be processed, making this test ideal for population-based screening [7].
The most used and better evaluated methods nowadays for CRC screening are those based on latex agglutination (OC-Sensor®, OC-Micro®, Eiken Chemical Co., Ltd, Japan; FOB-Gold® Sentinel Diagnostics®, Milan) or magnetized gelatin particles (Magstream1000®, Fujirebio Inc.,Tokyo, Japan).
Effectiveness of FOBT-based CRC screening in the the average-risk population
As previously commented, multiple studies have demonstrated that CRC screening with cFOBT in the average-risk population significantly reduces CRC mortality [8]. So far, there are no data available on the effect of FIT in CRC mortality or incidence. However, several diagnostic test studies have compared cFOBT and FIT for CRC and advanced adenomas detection. These studies have shown that FIT is more sensitive and specific for CRC and advanced adenomas detection and is a cost effective screening test [9]. On this basis, CRC screening programmes are based mainly on FIT.
Efficacy of FOBT CRC screening in the familial-risk population
Evidence on the best screening strategy in this population is limited and its quality is low [10, 11]. At present, it is unclear which is the best screening strategy in individuals with a FDR with CRC [12]. Clinical practice guidelines recommend more aggressive screening than in the average-risk population, based on studies that have shown that endoscopic screening reduces CRC incidence and mortality in individuals with a FDR with CRC [2, 6]. Screening is recommended from 40 years or 10 years before the youngest proband. However, this approach has an empirical basis and no prospective controlled study has compared different screening strategies in this population. Furthermore, colonoscopy is an invasive technique that requires sedation, is associated with rare but serious complications (perforation or hemorrhage), requires a substantial financial investment in equipment and expertise and has a limited adherence.
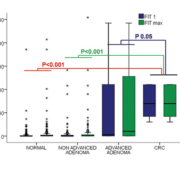
Data on the utility of FOBT for CRC screening in this population are scarce. Our group has recently evaluated FIT diagnostic accuracy for advanced colorectal neoplasia (CRC or advanced adenoma) in asymptomatic individuals with at least one first-degree relative with CRC submitted to a colonoscopy [13]. Patients with an advanced neoplasia had a fecal hemoglobin concentration statistically higher than those without an advanced neoplasia. Fecal hemoglobin concentration was significantly higher among individuals with CRC or advanced adenomas when compared with subjects with non-advanced adenomas or without neoplasia. In contrast, no significant differences were found between individuals with CRC and advanced adenomas as shown in Figure 1. Diagnostic accuracy for CRC detection was high, reaching 100% at a fecal hemoglobin threshold below 115 ng/ml. On the other hand, depending on the number of determinations and the positivity threshold cut-off used sensitivity for advanced neoplasia detection ranged between 34.38 and 50% and specificity between 92.66 and 98.72%. In fact, analysing two samples did not improve diagnostic accuracy and, instead, increased the number of colonoscopies needed to detect a CRC or an advanced neoplasia and the costs per lesion detected [Fig. 2]. These data suggest that FIT is an adequate CRC screening test in this population. However, FIT as a screening method must be evaluated in prospective controlled studies designed to determine CRC mortality reduction in the long term.
Conclusion
The reduction in mortality in FOBT-based CRC screening programmes and the improved sensitivity of immunochemical tests for CRC and advanced adenomas detection makes FIT CRC screening programmes a cost effective strategy. Preliminary data show that FIT is equally effective in the familial-risk population. Thus, FIT could be an adequate CRC screening technique in this population.
References
1. Ferlay J, Shin H-R, Bray F, Forman D, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127(12): 2893–2917.
2. Levin B, Lieberman DA, McFarland B, Andrews KS, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008; 134: 1570–1595.
3. Baglietto L, Jenkins MA, Severi G, Giles GG, et al. Measures of familial aggregation depend on definition of family history: meta-analysis for colorectal cancer. J Clin Epidemiol. 2006; 59(2): 114–124.
4. Butterworth AS, Higgins JPT, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer 2006; 42(2): 216–227.
5. Mandel JS, Church TR, Bond JH, Ederer F, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. NEJM 2000; 343(22): 1603–1607.
6. Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010; 375(9726): 1624–1633.
7. Quintero E. [Chemical or immunological tests for the detection of fecal occult blood in colorectal cancer screening?]. Gastroenterología y hepatología 2009; 32(8): 565–576.
8. Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008; 103(6): 1541–1549.
9. Guittet L, Bouvier V, Mariotte N, Vallee JP, et al. Comparison of a guaiac based and an immunochemical faecal occult blood test in screening for colorectal cancer in a general average risk population. Gut 2007; 56(2): 210–214.
10. Dove-Edwin I, Sasieni P, Adams J, Thomas HJW. Prevention of colorectal cancer by colonoscopic surveillance in individuals with a family history of colorectal cancer: 16 year, prospective, follow-up study. BMJ 2005; 331(7524): 1047.
11. Gimeno-García AZ, Quintero E, Nicolás-Pérez D, Hernández-Guerra M, et al. Screening for familial colorectal cancer with a sensitive immunochemical fecal occult blood test: a pilot study. Eur J Gastroenterol Hepatol. 2009; 21(9): 1062–1067.
12. Winawer S, Fletcher R, Rex D, Bond J, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology 2003; 124(2): 544–560.
13. Castro I, Cubiella J, Rivera C, González-Mao C, et al. Fecal inmunochemical test accuracy for colorectal cancer and advanced neoplasia detection in familial risk colorectal cancer screening. Int J Cancer 2013; doi: 10.1002/ijc.28353 (Epub ahead of print).
The authors
Inés Castro MD and Joaquín Cubiella* PhD
Department of Gastroenterology, Complexo Hospitalario Universitario de Ourense, Ourense, Spain
*Corresponding author
E-mail: Joaquin.cubiella.fernandez@sergas.es