Functional evaluation of chemistry analyser
The aim of this study was to assess the practicability and evaluate the analytical characteristics of the Mindray BS-2000M, a new automatic chemistry analyser, manufactured by Shenzhen Mindray Bio-Medical Electronics Co., Ltd (Mindray Shenzhen, China).
The evaluation involved 21 clinical chemistry parameters measured using indirect potentiometry, spectrophotometric and immunoturbidimetric assays.
Spectrophotometric assays
Alanine aminotransferase (ALT, according to IFCC with-out pyridoxal phosphate), aspartate aminotransferase (AST, according to IFCC method), gamma-glutamyltransferasa (GGT, according to Szasz), total bilirubin (TBIL, Diazotized sulfanilic acid method), calcium (Ca, Arsenazo III method), creatine kinase (CK, IFCC method), creatinine (Crea, modified Jaffe method), glucose (Glu, Hexokinase method), high density lipoprotein-cholesterol (HDL-C, direct method), magnesium (Mg, Xylidyl blue method), phosphorus (P, Phosphomolybdate method), total cholesterol (TC, Cholesterol oxidase – Peroxidase method), triglycerides (TG, Glycerokinase Peroxidase – Peroxidase method), total protein (TP, Biuret method), uric acid (UA, Uricase-Peroxidase method), iron (Fe, Colorimetric assay-Ferrozine), α-amylase [α-AMY, substrate: 4, 6- ethylidene-(G7)-1, 4-nitrophenyl-(G1) –α, D-maltoheptaoside (EPS-G7), enzymatic colorimetric assay according to IFCC method] and urea (Urea, Urease-glutamate dehydrogenase).
Immunoturbidimetric assays
Apolipoprotein A1 (ApoA1).
Indirect potentiometry assays (ISE)
Sodium (Na), potassium (k) and chloride (Cl).
Analytical evaluation
Among all the available parameters to verify the Mindray BS-2000M analytical performance, those which are more frequently requested in routine clinical practice were selected (e.g., glucose, creatinine, total protein).
An imprecision study was carried out according to the CLSI EP5-A2 guideline [1]. The within run imprecision was evaluated using two control materials. Final results were expressed as coefficient of variation (CV%). We checked that these CV satisfied the allowable maximum imprecision based on biological variability [2]. These data were taken from the listing of biological variation by Ricos et al [3], recently updated in 2012.
The inaccuracy study was done according to the CLSI EP9-A2 guideline [4], measuring at least 40 patient samples for the two analysers (Mindray BS-2000M and ADVIA 2400 Siemens Healthcare Diagnostics, USA) for 5 days.
In the inaccuracy study the mean bias and 95% confidence interval (CI) was calculated with the Bland-Altman plot analysis, and the linear regression was assessed using Passing-Bablok regression method [5-6].
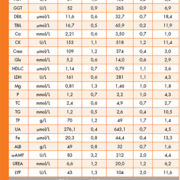
The results of the imprecision study [Table 1] showed that for all the parameters imprecision was lower than the maximal allowable applying to biological variability based criteria, with the exception of sodium (0.7%) and chloride (0,8%) in control 1 and total proteins (1,7%) in control 2. Nevertheless, these three parameters fulfill the commonly used “State of the art” criterion. According to this criterion, the maximal allowable imprecision for physiological concentrations must be less than the 0.20 fractile of the between-run imprecision (CV) of the laboratories in a external quality assessment scheme (7). The CV% limit for these three parameters following this approach would be 0.9% for Na, 1.6% for Cl and 1.7% for TP.
In the comparison study, a close correlation was observed for all parameters studied (r range: 0.92 – 1.00) [Table 2]. It is noted that there were no significant differences for 11 of the 21 parameters studied. For the other parameters statistically significant differences were found but, except for creatinine, those differences were not considered to have a clinical significance. The constant and proportional differences may be due to different standardization of both procedures. Traceable calibration materials should be used related to the reference method and also switchable materials that reveal the degree of deviation of multiple methods with respect to the true value should be used [8].
References
1. Clinical and Laboratory Standards Institute. Evaluation of precision performance of quantitative measurement methods; approved guideline -second edition. CLSI document EP5-A2. Wayne, PA:CLSI, 2004.
2. Fraser CG, et al. Proposed quality specifications for the imprecision and inaccuracy of analytical systems for clinical chemistry. Eur J Clin Chem Clin Biochem 1992; 30: 311.
3. Ricos C, et al. Desirable quality specifications for total error, imprecision, and bias, derived from biological variation. http://www.Westgard.com/biodatabase1.htm. Accessed on February 15, 2013.
4. Clinical and Laboratory Standards Institute. Method comparison and bias estimation using patient samples; approved guideline – second edition. CLSI document EP9-A2. Wayne, PA: CLSI, 2002.
5. Bland JM, et al. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307.
6. Passing H, et al. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part I. J Clin Chem Clin Biochem 1983; 21: 709.
7. Sebastian-Gámbaro, et al. An improvement on the criterion of the state of the art to estimate the maximal allowable imprecision. Eur J Clin Chem Biochem. 1996; 34: 445.
8. Documento de consenso. Sociedad Española de Bioquímica Clínica (SEQC) y Sociedad Española de Nefrología (SEN). Recomendaciones sobre la utilización de ecuaciones para la estimación del filtrado glomerular en adultos. Química Clínica 2006; 25: 423.
The author
Dr. Jose Luis Bedini,
Hospital Clinic I Provincial De Barcelona,
Barcelona,
Spain
MINDRAY