Go with the flow? Use of flow cytometry for routine leukocyte differential
Recently several clinical laboratories have reported antibody cocktails to perform leukocyte differentiation for routine screening. Distinct advantages over microscopic leukocyte differentiation are the large number of counted cells (tens of thousands) and objective immunological definition of the cell types. Here we review the published protocols and their usefulness for a routine setting.
by Dr G-J van de Geijn, Dr M. Beunis, Dr H. Janssen and Dr T. Njo
Differential white blood cell counting
Differential white blood cell count (dWBC) is an important and widely applied diagnostic test. The current generation of routine cell counters automatically produce a fast and reliable dWBC for most non-pathological samples. If dWBC results are aberrant or there are technical issues, the routine haematological analyser typically ‘flags’ a sample, and microscopic differentiation is mandatory. This dogma has been challenged by recent publications from independent groups. Although technically different, the approaches these groups have in common is that they each use a single flow cytometric tube for dWBC. This makes an implementation, which may be technically complicated and expensive, potentially feasible for clinical practice. Here we review the relative merits of the different flow cytometric approaches and attempt to position flow cytometric dWBC in clinical practice.
To appreciate the merits and disadvantages of the new flow cytometric approaches against the current microscopic practice, one must realise that leukocyte identification by flow cytometry is fundamentally different from microscopy. For example, microscopy can not differentiate lymphocyte subsets (B, T and NK cells), which are essentially defined immunologically. Flow cytometry can not replicate the microscopic classification of myeloid precursors because antigen expression in myeloid differentiation follows a different path from the microscopic phases. Although some of the dogmas for the interpretation of the microscopic leukocyte differentiation are more ‘practice-based’ than ‘evidence-based’, microscopy has the distinct advantage of a long history in clinical practice. A significant amount of training is required to ensure and maintain sufficient expertise among technicians to offer reliable round-the-clock service for microscopic dWBC. Due to the low number of cells counted (100-200) and the unequal distribution of cells on the slide, statistical variation and inter-observer differences are significant, well known disadvantages of microscopy [1,2].
Advantages of flow cytometry over microscopy are the large number of cells that are analysed (tens of thousands and more) and the objective immunological definitions of the different leukocyte types, using monoclonal antibodies defined by the international Human Leukocyte Differentiation Antigens (HLDA) classification system. This facilitates a more robust and evidence-based approach. In addition, different and more classes of leukocytes can be defined using flow cytometry compared to microscopy, providing growth potential for defining new cell populations for diagnosing and following up clinical diagnoses. Disadvantages of flow cytometry are increased costs of equipment, and that it is currently not used in many first-line haematology labs.
A new position for flow cytometry in routine clinical practice?
In the current clinical diagnostic setting, flow cytometry is almost exclusively performed in specialised laboratories during office hours, mainly as an established technique in leukaemia and lymphoma diagnostics. In routine haematological practice flow cytometry is not widely adopted. It is sometimes used as a reference method for quantifying leukocytes, erythroblasts and platelets during validation of a routine cell counter. Besides quantifying platelets and CD4 cell counts in the Celldyn-4000 and Sapphire routine cell counters, there is currently no widely adopted application in the routine laboratory. For leukocyte differentiation flow cytometry is mentioned in the CLSI guidelines as a candidate reference method for leukocyte differentiation. However the current reference method is still microscopic differentiation [3].
Flow cytometry
Flow cytometry uses specific monoclonal antibodies to detect cellular characteristics. These antibodies are labelled with fluorescent dyes emitting light at different specific wavelengths. Cell suspensions stained with a cocktail of antibodies can be analysed rapidly by flow cytometry which runs the cells past a laser. Light scatter and fluorescent signal are detected in different channels to give information on cell type, granularity and maturity of cells. Using combinations of these parameters the different cell types are detected in two-parameter dot-plots by so-called gates.
Flow cytometric differential white blood cell counting
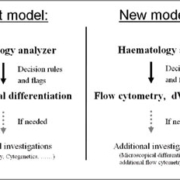
In recent years several labs have reported antibody cocktails combined with acquisition protocols to perform flow cytometric dWBC [4-7]. The goal of these publications is to test if flow cytometric dWBC can be performed in a single tube as a screening tool for samples flagged for review by the haematology analyser [Figure 1]. Flagged samples are tested first by flow cytometry, which may reduce the number of microscopic differentiations required. These protocols have in common that they use a single tube approach requiring a small amount of blood and a cocktail of antibodies to determine an extended dWBC by using flow cytometry. Which leukocyte populations are defined, the number of leukocyte populations and the strategy used to define them differs [Table 1]. The main features of these protocols are discussed below.
Faucher and colleagues were the first to report their antibody cocktail, discriminating 12 different cell populations using a 6-marker/5-colours protocol [4]. This is the only cocktail using CD2, which enables identification of mature T-cells as well as T-blasts. This can be an advantage in detecting T-ALL with CD34- CD3- blasts. Another difference with the other cocktails is the use of CD294 to positively identify basophils, eosinophils and T-cells. The description of the lymphocyte subsets is incomplete as NK and T-cells cannot be discriminated. Although there is no general blast-marker to aid blast detection, blasts are detected and classified as T-lineage, B-lineage, monocytic or other blasts. NRBCs and plasma cells are not detected.
Using this antibody cocktail with a slightly adapted gating strategy, the first routine application, with flow cytometric dWBC integrated in the workflow of a haematology laboratory was published [8]. Samples flagged by the haematology analyser were analysed by flow cytometry before microscopy. Flow cytometer acquisition software that automatically adapts the gates to fit the different leukocyte populations and an automated pipetting station were used as technical aids. The authors show that this approach reduces the number of microscopic differentiations, manual hands-on time and turn-around-time. A group from Korea tested this cocktail with automatic gating software on a set of leukopenic samples, known to give problems with a reliable microscopic dWBC [9]. Both groups report that the gates were set correctly by the automatic software in >75% of the samples.
The cocktail reported by Bjornsson et al differentiates all nucleated cells in 11 categories using 6 markers and DRAQ5 staining with a 5-colour flow cytometer [5]. This protocol cannot discriminate between T and B-lymphocytes and uses CD203 to facilitate basophil detection. In contrast to the other cocktails, when the sample is diluted and re-measured using a low acquisition rate, CD36 can also be used to detect platelets.
Cherian and colleagues describe a 10 markers/8-colour cocktail including Hoechst staining to detect 12 leukocyte categories and NRBCs [6]. Strong points of their approach are the inclusion of CD34/117 for more robust blast detection, resulting in good correlations with microscopy. Furthermore CD33/64 is used for positive definition of monocytes and eosinophils, CD123 for basophils, CD38 for plasma cells and Hoechst to quantify NRBCs. No positive defining marker for T-cells is included.
Recently we reported our 10 marker/5-colour flow cytometric dWBC cocktail called Leukoflow [7]. Compared to the other cocktails, this cocktail uses the largest number of antibodies on a 5 colour machine. Although behind the scenes this requires a complex gating strategy to define the populations, the manual gating is not too difficult. Compared with the other methods, this assay is the most complete in defining lymphocyte subsets. Using CD3, CD19, CD16, CD56 and CD4 all lymphocyte subsets can be defined, including CD4-positive T-cells, except for the double positive CD3 and CD8 cells. CD138 is used to detect plasma cells. CD34 aids detection of blasts which can be further subdivided into blasts of the B-lymphoid, T-lymphoid or myeloid lineage. There is no positive marker for basophils. NRBCs can be quantified using a separate staining with DRAQ5 and antibodies.
Correlations between flow cytometry and cell counter/microscopy
The results of each of these reported flow cytometric protocols were compared with the results from haematology analysers and microscopy for sets of normal and abnormal blood samples. For normal, implicit, blood samples there are no real differences in the correlations between flow cytometry and microscopy for the different cocktails. In general, the correlations for neutrophils, lymphocytes and eosinophils are very good (>0.9) whereas the correlation for monocytes is lower (0.63-0.86) and the correlation for basophils is the poorest (0.29-0.70). To assess how these protocols compare when differentiating leukocytes in abnormal blood samples (e.g. containing plasma cells, blasts or immature granulocytes), these protocols should be compared on the same samples. This has not yet been reported in literature.
Additional clinical value of flow cytometric dWBC
Given the fact that different and more leukocyte populations can be identified with flow cytometric dWBC the question arises as to whether this additional information also has additional diagnostic value. Several examples of this have already been demonstrated. Roussel et al report efficient use of the ratio of T and B lymphocytes to discriminate B lymphoproliferative disorders in a random selected group of 349 with WBC >4×109/L [8]. This indicates that other flow cytometric dWBC methods that measure B- and T-lymphocytes, such as the ones reported by Cherian et al and our group can also use this [6,7]. Faucher et al demonstrated that in patients without known haematological disease flow cytometric dWBC can help to detect those with inflammatory syndrome (acute bacterial infection, heart failure, cancer, systemic disease) by their enhanced count of CD16-positive monocytes [4]. CD16-positive monocytes are found in nearly all inflammatory diseases [10]. CD16 positive monocytes are modulated during conditions such as atopic eczema, malaria infection and sepsis [11,12]. Information on CD16-positive monocytes can also be obtained with the other antibody cocktails using CD14, CD36 or CD33+CD64 to define monocytes.
The cocktail by Cherian et al contains CD64, which is reportedly upregulated on granulocytes during infection or sepsis [6,13]. Proper validation of the added clinical diagnostic value of all these parameters requires further investigations comparing patient cohorts homogeneous for the conditions mentioned above with the appropriate control patients.
Conclusion
All studies reported so far demonstrate that flow cytometric dWBC is technically feasible, and its results in general correlate well with the other known dWBC techniques. In order to compare the performance of these cocktails with each other it is crucial that they are compared on the same sample set. To our knowledge, such a comparison has not been published yet. Since all publications report good correlations between their flow cytometric dWBC and other methods for dWBC, we expect no big differences between the different cocktails for normal samples. For abnormal samples there will be differences due to the different composition of the cocktails. For implementation in a routine setting as a screening technique in between the haematology analyser and microscopic smear review, an automatic gating protocol is a significant advantage. This is only available for one of the reported methods so far. However, in order to make it a robust system suitable for use by a large group of technicians with 24/7 service, development of a flagging system that detects abnormalities/errors in the automated gating, as is present on haematology analysers, is a must. Unfortunately this is not available for any of the reported flow cytometric protocols yet, but it deserves significant attention to make this promising technique attractive for routine laboratories.
References
1. Pierre RV. Peripheral blood film review. The demise of the eyecount leukocyte differential. Clin Lab Med 2002;22(1):279-97.
2. Ruemke CL. The statistically expected variability in differential leukocytes counting In: Koepke JA, editor. Differential Leucocytes Counting. CAP Conference Aspen College of American Pathologist 1977; p 39-46.
3. Koepke JA, Van Assendelft OW, Brindza LJ, Davis BH, Fernandes BJ, Gewirtz AS, Rabinovitch A. Reference Leukocyte (WBC) Differential Count (Proportional) and Evaluation of Instrumental Methods; Approved Standard-Second Edition. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute 2007; 1-35 p.
4. Faucher JL, Lacronique-Gazaille C, Frebet E, Trimoreau F, Donnard M, Bordessoule D, Lacombe F, Feuillard J. ‘6 markers/5 colors’ extended white blood cell differential by flow cytometry. Cytometry A 2007;71(11):934-44.
5. Bjornsson S, Wahlstrom S, Norstrom E, Bernevi I, O’Neill U, Johansson E, Runstrom H, Simonsson P. Total nucleated cell differential for blood and bone marrow using a single tube in a five-color flow cytometer. Cytometry B Clin Cytom 2008;74(2):91-103.
6. Cherian S, Levin G, Lo WY, Mauck M, Kuhn D, Lee C, Wood BL. Evaluation of an 8-color flow cytometric reference method for white blood cell differential enumeration. Cytometry B Clin Cytom 2010;78(5):319-328.
7. van de Geijn GJ, van Rees V, van Pul-Bom N, Birnie E, Janssen H, Pegels H, Beunis M, Njo T. Leukoflow: multiparameter extended white blood cell differentiation for routine analysis by flow cytometry. Cytometry A 2011;79(9):694-706.
8. Roussel M, Benard C, Ly-Sunnaram B, Fest T. Refining the white blood cell differential: the first flow cytometry routine application. Cytometry A 2010;77(6):552-63.
9. Jo Y, Kim SH, Koh K, Park J, Shim YB, Lim J, Kim Y, Park YJ, Han K. Reliable, accurate determination of the leukocyte differential of leukopenic samples by using Hematoflow method. Korean J Lab Med 2011;31(3):131-7.
10. Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol 2007;81(3):584-92.
11. Novak N, Allam P, Geiger E, Bieber T. Characterization of monocyte subtypes in the allergic form of atopic eczema/dermatitis syndrome. Allergy 2002;57(10):931-5.
12. Skrzeczynska J, Kobylarz K, Hartwich Z, Zembala M, Pryjma J. CD14+CD16+ monocytes in the course of sepsis in neonates and small children: monitoring and functional studies. Scand J Immunol 2002;55(6):629-38.
13. Davis BH, Olsen SH, Ahmad E, Bigelow NC. Neutrophil CD64 is an improved indicator of infection or sepsis in emergency department patients. Arch Pathol Lab Med 2006;130(5):654-61.
The authors
Dr Gert-Jan van de Geijn, Dr Marlène Beunis, Dr Hans Janssen and drs Tjin Njo, MD.
Department of Clinical Chemistry (KCHL),
Sint Franciscus Gasthuis,
Kleiweg 500,
3045 PM Rotterdam,
The Netherlands.
e-mail: g.vandegeijn@sfg.nl