High-sensitivity success for achy breaky hearts
In May 2018, a rapid assessment and risk stratification pathway was implemented in Unscheduled Care for patients with suspected acute coronary syndrome, using high-sensitivity (hs) Troponin T. Patients could be ruled out and discharged within one hour of presentation to the Emergency Department. The assay was validated and its safety and clinical effectiveness assured through a retrospective audit. A further audit two years after pathway implementation demonstrated that the pathway was being used appropriately and had potentially resulted in both financial savings to the local Clinical Commissioning Group and significantly saved admissions and bed days for the Acute Hospital Trust. Overall, hs Troponin T can be used in a rapid one-hour pathway for the safe and effective risk stratification in patients presenting to Unscheduled Care departments.
Background
Each year in the UK over 80 000 patients are admitted to hospital with acute coronary syndrome (ACS), which classically presents as chest pain or discomfort. ACS is subdivided into ST-elevation myocardial infarction (STEMI) and non-ST-elevation ACS (NSTE-ACS); the latter may be categorized as non-ST elevation myocardial infarction (NSTEMI) or unstable angina (UA) [1].
The fourth universal definition of myocardial infarction is an acute myocardial injury (defined as a Troponin concentration above the 99th centile) alongside evidence of acute myocardial ischemia. It is essential that patients are diagnosed promptly and accurately in order to commence effective treatment and reduce morbidity and mortality.
Current practice
Traditionally, patients who present to the Emergency Department (ED) with symptoms suggestive of NSTEMI, but who are considered low risk, have blood taken for cardiac Troponin I or T analysis as part of a six-hour pathway. Samples are taken at presentation and then repeated six hours later to identify changes in Troponin concentration. In practice, this pathway results in hospital admittance of patients in order to avoid breaching NHS England’s four-hour ED waiting time target, which may be unnecessary in patients in whom the Troponin result is subsequently negative and who can otherwise be safely discharged.
Algorithms using high-sensitivity
Troponin Newly developed high-sensitivity (hs) Troponin assays have allowed assessment of these cardiac biomarkers at earlier time points in disease progression than the six-hour pathway. A number of studies have been published that present evidence that a shorter pathway, with earlier blood sampling, is accurate and safe in correctly classifying patients based on Troponin concentrations that are lower than the 99th centile [2–4]. Such pathways were developed to allow early rule-out of suspected NSTEMI and to support the safe discharge of patients from ED without hospital admittance to wait for the results of further Troponin tests.
Development of a zero-hour/one-hour pathway
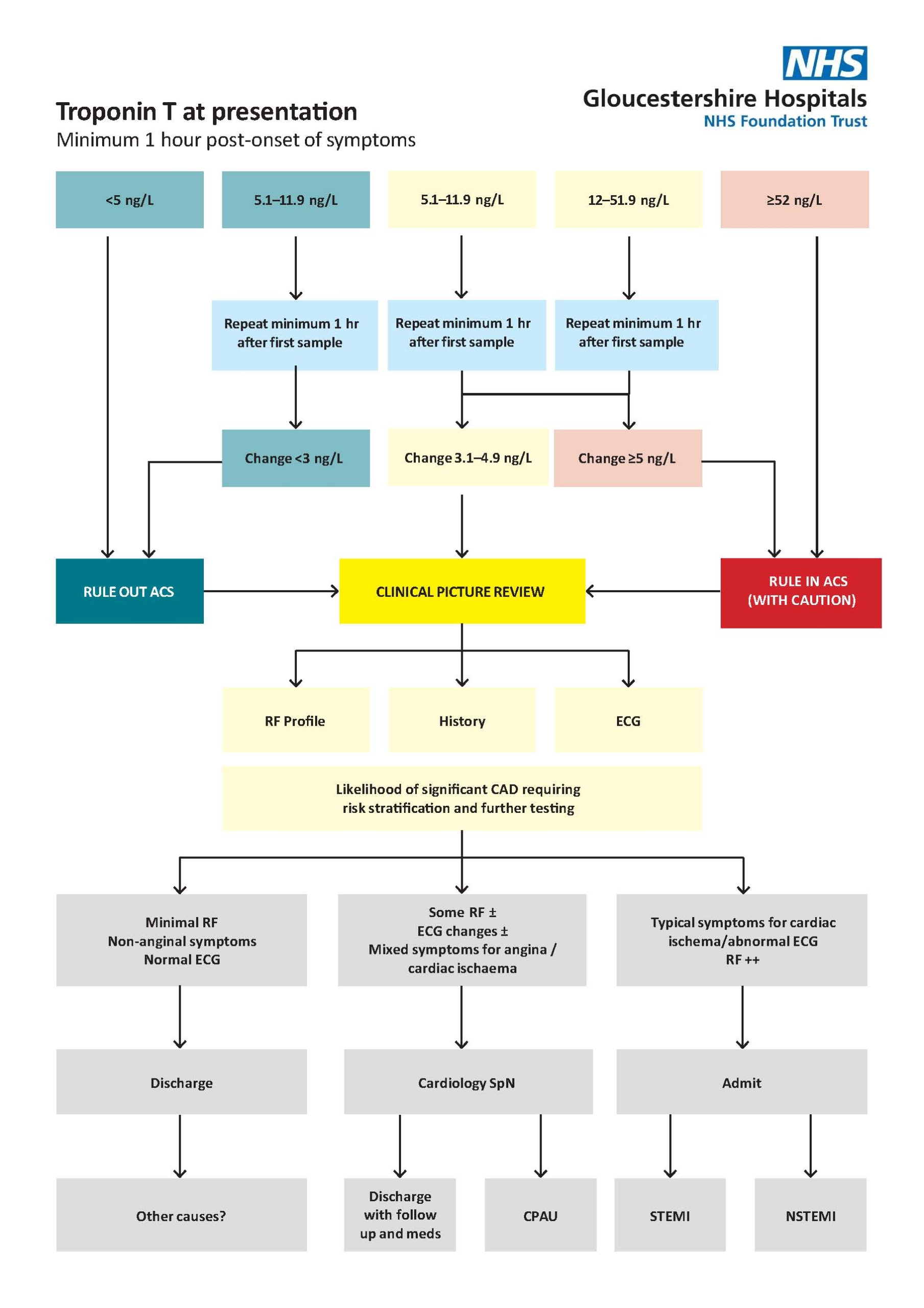
Based on expert recommendations, the Biochemistry and Cardiology departments worked together to assess the viability of the ‘zero-hour /one-hour’ pathway for patients presenting to Unscheduled Care in Gloucestershire Hospitals NHS Foundation Trust [3]. The algorithm was based on taking a blood sample for Troponin T analysis at presentation in the ED (a minimum of three hours post-onset of chest pain) with a follow-up sample taken one hour after the first sample. The patient could be discharged based on the result of the presentation sample, if below the rule-out cut-off concentration of 5 ng/L, or after review of the second sample, if the change between the two results was within defined concentrations. The pathway is shown in Figure 1.
The Elecsys® Troponin T hs assay was validated to measure down to 14 ng/L, but the European Society of Cardiology (ESC) zero-hour /one-hour pathway relied on accurate determination of Troponin T well below this cut-off value, down to the limit of detection (the lowest analyte concentration reliably distinguished from the limit of blank) of 5 ng/L [3]. Thus, the first challenge facing the Biochemistry laboratory was validation of the Elecsys® Troponin T hs assay to demonstrate acceptable imprecision at this much lower concentration. The laboratory assessed analytical performance by repeated measurement of pooled patient samples across the three Elecsys® analysers, followed by calculation of the mean, standard deviation and coefficient of variation (CV). CVs of less than 4% indicated that performance at the limit of detection was acceptable for the use of the ESC zero-hour/one-hour pathway and that the assay could distinguish the small changes in concentration necessary for patient discharge or admittance [3].
Consideration of patient safety was at the heart of the pathway. Once analytical performance was assured, a retrospective audit was conducted to compare patient outcomes in the six-hour pathway in place at the time against the potential ESC pathway. The audit found that requesting patterns in the ED were very variable, with frequent inappropriate requesting and inconsistent adherence to the six-hour pathway. However, the audit concluded that the ESC pathway would have been a safe and effective algorithm for ruling out NSTEMI in these low-risk patients, as all patients were alive and well after a period of two years and the one patient that represented with chest pain within 30 days of the first episode had a non-significant Troponin T result once again. The effect of analytical bias was also considered, as the assay was run on three analysers across two sites, but this was insignificant and would not have affected patient outcomes. Quality control was sourced to ensure assay performance was acceptable near the limit of detection (5 ng/L).
Following the establishment of the safety of the Troponin T algorithm, the clinical and patient-management aspects were agreed by a multidisciplinary team that included representation from Cardiology, ED and Pharmacy, as well as Biochemistry. It was essential that the clinical teams could support patient throughput and that clear management pathways were defined. The new ACS pathway was launched in the Trust in May 2018.
Auditing the new pathway
Two years after its introduction, a re-audit was undertaken to assess patient safety and health-professional compliance with the pathway. The vast majority of the requests were found to be appropriate, although differences were seen between the two Trust hospital sites. Inappropriate requests were noted to include patients presenting with seizures, arthritis and overdose. Regardless of whether the initial Troponin T request was appropriate, the pathway was then followed correctly in over two-thirds of cases. By this point, nearly half of patients could be ruled out as having a non-ACS cause of their symptoms; however, the decision to admit would have been based on the patient’s overall clinical picture, taking into account both their Troponin T concentration(s) and any other cardiac or non-cardiac reasons for their presentation to ED. A third of patients had Troponin T results that indicated that they could be discharged according to the pathway shown in Figure 1. However, over a third of these were admitted owing to their overall clinical picture, for reasons such as poisoning and malignancy. Of the patients who were subsequently discharged based on a rule-out Troponin T result, all were still alive two years after their presentation in ED. The majority had no further incidences of chest pain, and for those that did have repeated occurrences, the subsequent Troponin T results overwhelmingly indicated non-ACS causes for their symptoms. The risk stratification and follow-up of the remaining patient was appropriate.
Benefits of the new pathway
Cost savings
The audit showed that the equivalent of 11.5 patients per day could be safely discharged, who would otherwise have been admitted to wait for their Troponin T results. This would save the local Clinical Commissioning Group (CCG) nearly £850 000 each year in admission fees that would otherwise be paid to the Acute Hospital Trust.
Bed day savings
The audit showed that the Acute Hospital Trust would save 11.5 beds every day by not admitting patients who could otherwise be discharged. This is equivalent to over 4000 bed days every year.
Conclusion
The pathway represented significant savings both to the Acute Hospital Trust and CCG compared to the previous six-hour pathway in savings on the admission fee and in bed days. The follow-up audit supports the continued use of the risk stratification pathway in Unscheduled Care for patients with suspected ACS or who have cardiac sounding chest pain. As one of the earliest adopters of the pathway, the scientists and specialist nurses involved were subsequently able to contribute to the National Institute for Heath Care and Excellence (NICE) Adoption Support Resource to support other Trusts implementing an early rule-out protocol [5].
Figure 1. The rule-out algorithm in use at Gloucestershire Hospitals NHS Foundation Trust for rapid assessment and risk stratification of patients with suspected acute coronary syndrome (ACS) or who have cardiac sounding chest pain
The pathway was developed by Biochemistry, Cardiology and ED based on the ESC 2015 guidance [3].
CPAU, Chest Pain Assessment Unit; ECG, electrocardiogram; RF, risk factors; SpN; Specialist Nurse; (N)STEMI, (non)-ST-elevation myocardial infarction.
Acknowledgments
Dr Peter Scott, Cardiology Consultant
Sister Alison Halliday, Senior Specialist Nurse, Cardiology
Jenny Deane, Cardiac Chest Pain Specialist Nurse, Cardiology
Dr Mark Allen, ED Consultant
Dr Marianne Gilllings, ED Consultant
Eve Olivant, Matron, Cardiology and Respiratory
The authors
Sadie Thomas PhD and Emma Stevenson
Clinical Biochemistry, Gloucestershire Hospitals NHS Foundation Trust, Gloucester, GL1 3NN, UK
*Corresponding author
E-mail: Sadie.Thomas@somersetft.nhs.uk
References
- Kotecha T, Rakhit RD. Acute coronary syndromes. Clin Med (Lond) 2016;16(Suppl 6):s43–s48 (https://www.rcpjournals.org/content/clinmedicine/16/Suppl_6/s43).
- Diagnostics Guidance [DG15]. Myocardial infarction (acute): Early rule out using high-sensitivity Troponin tests (Elecsys Troponin T high-sensitive, ARCHITECT STAT High Sensitive Troponin-I and AccuTnI+3 assays). National Institute for Heath Care and Excellence (NICE) 2014 (https://www.nice.org.uk/guidance/dg15).
- Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. European Society of Cardiology 2015 (https://www.escardio.org/static-file/Escardio/Guidelines/Publications/ACS/2015_NSTE-ACS%20Gles-Web-Addenda-ehv320.pdf).
- Shah AS, Anand A, Sandoval Y et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet 2015;386(10012):2481–2488 (https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(15)00391-8/fulltext).
- Implementation support. Adoption support resource – insights from the NHS. NICE 2019 (https://www.nice.org.uk/guidance/mtg36/resources/adoption-support-resource-insights-from-the-nhs-pdf-8633184204229).