How the COVID-19 response was informed by – and informs – our battle against other global health crises
The COVID-19 pandemic has reinforced the critical importance of diagnosis and access to these services, both at the individual and at a population level. The pandemic has also impacted the delivery of diagnostics and care for other medical conditions, such as HIV/AIDS, but similarly, lessons learned with COVID-19 can also be applied to improve diagnostic services for other infectious diseases.
Introduction
If the public didn’t fully appreciate the importance of having robust diagnostics networks in place, the COVID-19 pandemic has likely been a swift education. We’ve seen the challenges at nearly every step: sparse and unreliable testing in the early days of the pandemic; queues wrapping around blocks in cities throughout the pandemic for testing; chronically sold-out home test kits; and little access to testing sites in rural areas. These issues have been reminders of the importance of capable testing networks – not just for the public but also for those of us in the life sciences arena whose work involves bringing diagnostics to patients across the globe.
There are, of course, other viral pandemics and epidemics we’ve been fighting for much longer, HIV/AIDS being one of the most significant. The Joint United Nations Programme on HIV/AIDS (UNAIDS) has led the global effort to end HIV/AIDS as a public health threat: their updated goal for 2025 is the 95–95–95 target: 95% of all people living with HIV (PLHIV) will know their HIV status, 95% of all people with diagnosed HIV infection will receive sustained antiretroviral therapy, and 95% of all people receiving antiretroviral therapy will have viral suppression [1]. There’s been a lot of progress toward these goals – in many parts of the world, HIV/AIDS is no longer the death sentence it used to be, having been transformed into a more manageable chronic disease through better diagnosis and treatment [1].
But there are still great disparities in other countries. Two-thirds of the world’s PLHIV are in sub-Saharan Africa [1,2]. Even in the developed world, there are disparities, with inner city residents, for instance, having far less access to quick diagnosis and treatment than other subpopulations; the reasons for which are different in different countries. While the war against HIV/AIDS waged on, COVID-19 became a big disrupter to the various stages of progress. The pandemic has thrown new challenges upon existing challenges across nearly every area, from the lockdowns that prevented or discouraged people from seeking care to the massive global need for testing and vaccination [1,2]. The early days of the HIV/AIDS battle taught us the gruelling parameters of managing a pandemic. Combined with lessons learned from other existing pandemics, our knowledge has informed how we tackled COVID-19 – but COVID-19 will also shape how we’ll tackle all pandemics in the future.
A brief recap of HIV testing and monitoring
Generally, HIV is typically diagnosed through the detection of the viral nucleic acid, viral p24 antigen, or antibodies against HIV [1,3]. The antigen p24 is a structural protein found in the capsid of the HIV virus. After diagnosis, an individual generally starts antiretroviral therapy (ART), which reduces the viral particles in the blood (viral load) [1]. Routine monitoring is also needed: viral load testing determines whether ART is successful [1]. This has become the recommended strategy for monitoring a patient over time [1], and some countries have begun to use only this method [1]. However, cluster of differentiation 4 (CD4) cell count monitoring, which can be assessed using flow cytometry [4], is also extremely useful and is recommended in settings where viral load monitoring is not routinely available [1]. CD4 is a protein on the surface of T helper cells, which provides an indicator of a person’s immune status, and can be used to stage advanced HIV [1]. As HIV degrades the function of immune cells over time, PLHIV gradually become immune-deficient and more susceptible to certain types of cancer and opportunistic infections (OI) [1]. Once ART is started, a person’s immune status should improve, and CD4 count increase. However, if it decreases even after some months of ART, this suggests there is treatment failure, as the virus is developing resistance to the ART drugs [1]. Patients with very low CD4 counts are likely to need specific drugs to prevent specific OIs, in addition to their HIV treatment [1].
Self-testing
As mentioned, the COVID-19 pandemic changed many aspects of how healthcare was provided, including how and where diagnostics are performed [1,2]. Self-testing has become an important tool for managing COVID-19 in some countries: across Europe, Canada and India, free or very low-cost, readily available at-home testing kits have become a staple of diagnosis [1–3]. The USA has been slower to follow suit, and at-home tests have been in short supply, although the government has recently pledged US$650 million to boost rapid at-home testing efforts [5].
Self-testing can also play an important role in HIV diagnostics, and COVID-19 has proven its utility more generally [1]. Although it had existed previously, HIV self-testing was ramped up in many areas during the pandemic, including certain countries in Africa, in conjunction with the World Health Organization’s (WHO’s) extensive new guidelines for self-testing during the COVID-19 pandemic [1,2,6]. Self-tests, which use blood or saliva samples, can be picked up locally or ordered online and, with simple instructions, easily used by the individual [7,8]. During the COVID-19 pandemic, the availability of tests increased partly through partnerships with organizations, including pharmacies, retail stores, and health centres [7]. Support for self-testing was offered in various ways – in person, by phone, or even via instant messaging through apps such as WhatsApp – and a positive diagnosis triggered a cascade of events to link the individual to a medical professional for treatment [8]. Being ready to deploy self-tests kits for COVID-19, HIV, flu and other infections (as appropriate) will be an important part of prepared-ness in the future.
Telehealth
Similarly, the provision of pre-exposure prophylaxis (PrEP) plays an integral part in reducing HIV transmission [1]. And although this provision already exists, it needs to be ramped up to ensure that people who may have been exposed to HIV receive the best chance of an optimal prognosis, and that transmission of the virus is minimized. Key programmes for PLHIV during the COVID-19 pandemic, including those for PrEP provision, have fortunately proven resilient [9]. This has been largely driven by virtual telemedicine that has been successfully adapted to cope with the disruptions caused by the pandemic, particularly in the developed world, but also to a substantial extent in developing countries [9].
Telehealth, or telemedicine, has been an integral part of healthcare during the COVID-19 pandemic – from the large-scale virtual hospitals set up by large healthcare systems to the individual video and telephone appointments that smaller clinics offered their patients to reduce the risks associated with being seen in person [10,11]. Telehealth will likely continue to be a routine tool to monitor chronic health conditions and diagnose where possible, and can also be crucial as a means to maintain HIV care during the pandemic, including HIV diagnosis, monitoring for those who are already on ART, and even distribution of the HIV prophylactic Oral PrEP [12]. One recent study found that telemedicine use in sub-Saharan Africa rose during the pandemic, albeit unevenly, given certain challenges (internet connectivity, funding, public awareness) [11]. As more and more people have access to smartphones and other technologies, telemedicine will be a powerful tool to educate for prevention and monitor treatment of HIV/AIDS – and other health problems – moving forward.
Mobile health and point of care
The concept of meeting people where they are and providing care in the community has come a long way during the pandemic. Testing at mobile sites (e.g. vans) and community point-of-care (POC) locations were mainstays of COVID-19 testing across the globe [13]. The shift to community-level care was partly due to necessity (i.e. the sheer volume of COVID-19 testing needed), but also because it reduces barriers to seeking care for any health issue, and makes treatment and follow-up more likely. Expanding local care through mobile vans and POC has been a key effort in HIV care as well as other public health initiatives [14]. In its March 2021 update to HIV care guidelines, the WHO calls for an expansion in POC viral load HIV testing [15]. Studies have found that POC and mobile testing is effective: one study, carried out in South Africa, found earlier diagnosis and better linkage to care with mobile/POC CD4 testing [14]. Another study, which deployed mobile health vans provided by the Bill and Melinda Gates Foundation, found increases in viral suppression after a year’s supply of ART was given to individuals [15]. Deployment of testing and treatment sites in the community is another example of a practice that existed before, but whose value was reinforced during the pandemic and will continue long after.
Partnerships to build out networks
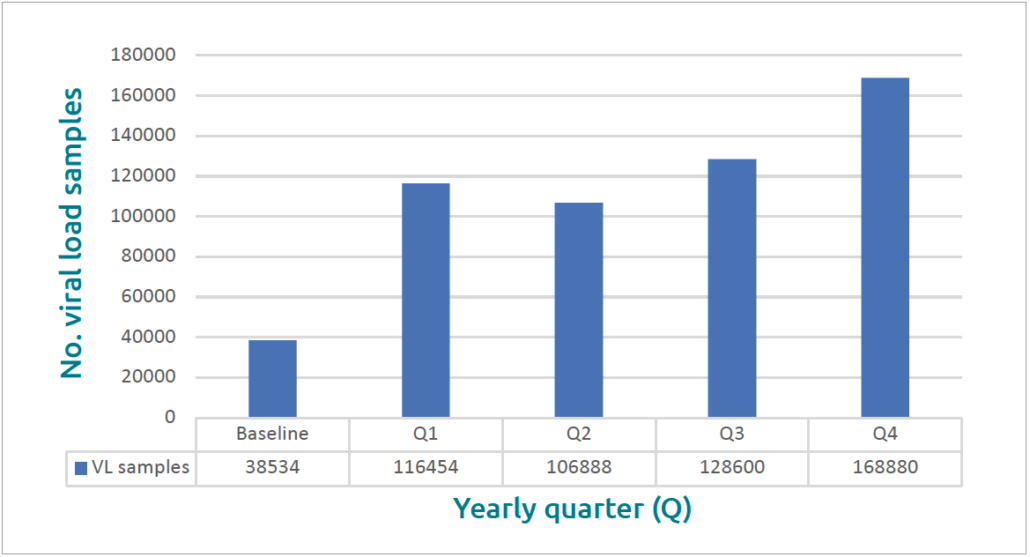
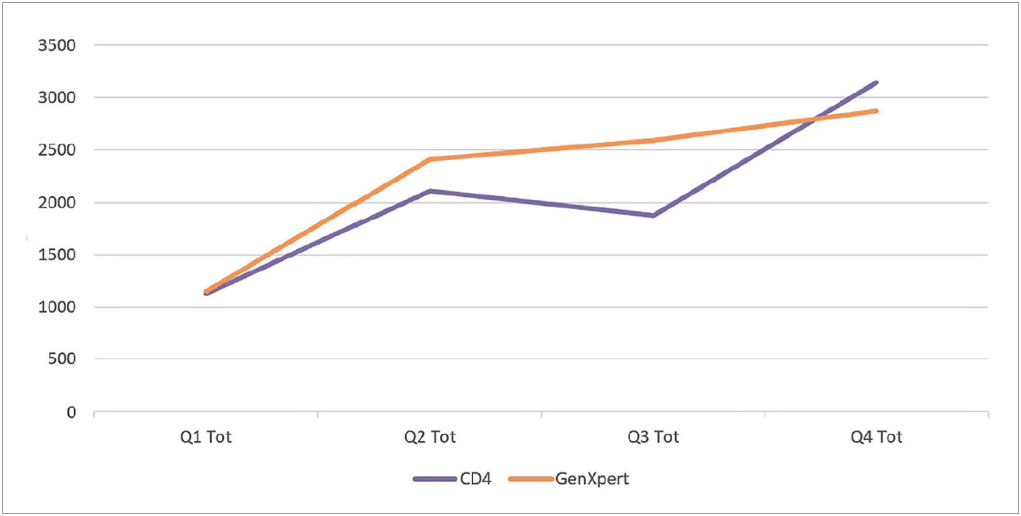
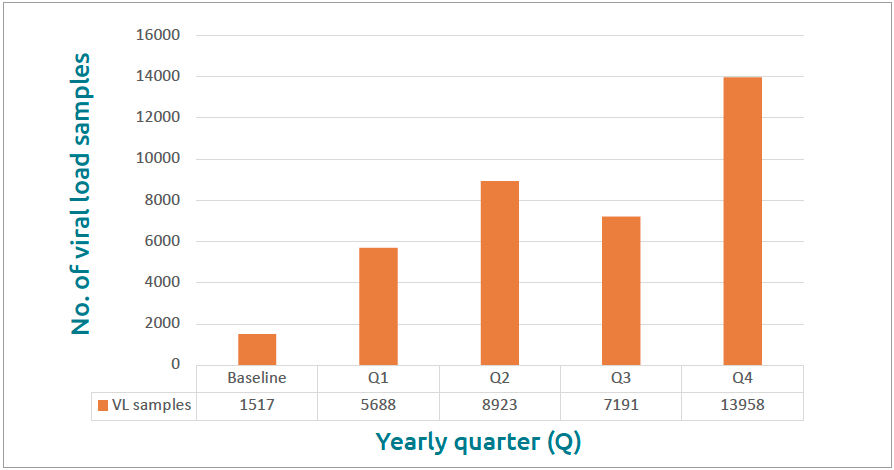
Finally, during the pandemic it’s become even clearer that some global health problems are so vast that no single organization can make a significant impact by itself. Tackling even a single disease can be overwhelming and seem, frankly, futile when attempted without strong partnerships with organizations that have common objectives. My colleagues’ and my work has centred on supporting existing HIV testing networks, namely in Uganda. The Ugandan Ministry of Health’s National Public Health Laboratory Service already had an extensive network in place, but contacted us to help fine-tune it and increase capacity. Together, we launched a pilot study to determine if it was possible to move away from a centralized approach and shift sample coordination to a regional level, expand reach, increase transportation efficiency, increase lab management efficiency, and reduce turnaround time, among others. Over 12 months, the number of viral load samples processed per quarter by larger hub sites rose by 130 346 over baseline to 168 880 in the fourth quarter, an increase of almost 350% (Fig. 1) [16]. The average number of samples that local sites sent on to the hubs for CD4 (Fig. 2) and viral load assessments increased by 800% (Fig. 3) [16]. This was a huge achievement for our entire team and an immensely gratifying outcome. Personally, it’s humbling to learn how simple ideas can have so much impact and to realize just how much room for improvement there was, and still may be, to provide better care for PLHIV and people who may have been exposed to HIV. Everyone involved in the study felt such a sense of accomplishment in playing their part to help prolong lives and prevent disease. Partnerships like this – between life sciences companies, public health departments, universities and non-profit organizations – have also been critical during COVID-19, in setting up telehealth and virtual hospitals, testing sites, mass vaccination sites and more. Organizations should be proactive in establishing partnerships that leverage the expertise of all involved and generate ambitious ideas that can be put into action and scaled.
Looking ahead
These are just a few ways in which existing initiatives have informed the COVID-19 response, and the COVID-19 pandemic has, in turn, deepened and accelerated those existing strategies. There are many other global public health issues that need attention and can benefit from what we’ve learned during the pandemic. My team’s work has also involved partnerships that support testing networks for pediatric blood cancers, including Burkitt’s lymphoma, a fast-growing and disfiguring form of cancer, which is often easily treatable once diagnosed. Synthesizing and applying information, not just across partners and disciplines but also across health initiatives, will also be key as we work even harder to improve health and health equity in the coming years. As COVID-19 transitions into an endemic rather than pandemic communicable disease, so too do we need to transition, and to embed more resilient and better prepared standards of care for PLHIV, particularly in regions like sub-Saharan Africa, where the burden is highest.
Digital illustration of HIV virus Source: Shutterstock
Figure 1. Number of viral load (VL) samples processed by quarter year (Q) at the hub: improvements over 12 months from before (baseline) and at the end of the project (Q4) Source: Mugerwa I, Boova T, Van Der Westhuizen, et al. Demonstrating how to improve operational efficiency in Uganda’s overall pathology service – part 3. Beckman Coulter Life Sciences 2021 [16].
Figure 2. Overall increases for sample volumes including CD4 and GeneXpert Source: Mugerwa I, Boova T, Van Der Westhuizen, et al. Demonstrating how to improve operational efficiency in Uganda’s overall pathology service – part 3. Beckman Coulter Life Sciences 2021 [16].
Figure 3. Number of viral load (VL) samples processed by quarter year (Q) at the lower facilities: improvements over 12 months from before (baseline) and at the end of the project (Q4) Source: Mugerwa I, Boova T, Van Der Westhuizen, et al. Demonstrating how to improve operational efficiency in Uganda’s overall pathology service – part 3. Beckman Coulter Life Sciences 2021 [16]
The authors
Samuel (Tony) Boova
Director Alliance Development, Global Flow Cytometry Business Unit, Beckman Coulter Life Sciences, Indianapolis, IN 46268, USA
E-mail: taboova@beckman.com
References
- Consolidated guidelines on HIV prevention, testing, treatment, service, delivery and monitoring: recommendations for a public health approach.
World Health Organization (WHO) 2021, 16 July (https://bit.ly/3JbjYm3). - Fact sheet – World AIDS day 2021. UNAIDS 2021 (https://bit.ly/3egsIJB).
- HIV testing overview. HIV.gov updated 2020, 25 September (https://bit.ly/3H1wOS8).
- Guidelines for HIV diagnosis and monitoring of antiretroviral therapy. WHO revised 2009 (https://bit.ly/3FkfVBG).
- Biden administration to invest $650 million in rapid diagnostic testing in latest action to increase access to tests.
U.S. Department of Health & Human Services (HHS.gov) 2021, 10 November (https://bit.ly/30QJQCx). - Considerations for HIV self-testing in the context of the COVID-19 pandemic and its response: an operational update.
HIV Self-Testing Africa Initiative 2020 (https://bit.ly/3J8FcRD). - Can I get an HIV test to use at home or in a private location? US Centers for Disease Control and Prevention (CDC).
Reviewed 2021, 16 July (https://bit.ly/3mn3ttG). - Hatzold K. Ramping up HIV testing in the era of COVID-19. Self-Care Trailblazer Group, Population Services International (psi.org) 2020, 6 May
(https://bit.ly/3H4pncP). - Boehmler A. Global AIDS strategy within the SARS-COV-2 pandemic. Medical Laboratory Observer 2021, 23 September (https://bit.ly/3ySygTT).
- Chitungo I, Mhango M, Mbunge E, et al. Utility of telemedicine in sub-Saharan Africa during the COVID-19 pandemic. A rapid review. Hum Behav Emerg Technol 2021: DOI: 10.1002/hbe2.297 (https://bit.ly/3ph6LjW).
- Continuing PrEP services for adolescents in Brazil despite COVID-19 disruptions. News. WHO 2020, 26 November (https://bit.ly/32b3Xw9).
- Attipoe-Dorcoo S, Delgado R, Gupta A, et al. Mobile health clinic model in the COVID-19 pandemic: lessons learned and opportunities for policy changes and innovation. Int J Equity Health 2020; 19(1): 73 (https://bit.ly/3sspnj4).
- Sloot R, Glenshaw MT, van Niekerk M, Meehan SA. Rapid point-of-care CD4 testing at mobile units and linkage to HIV care: an evaluation of community-based mobile HIV testing services in South Africa. BMC Public Health 2020; 20(1): 528 (https://bit.ly/3Jeb7QA).
- Updated recommendations on HIV prevention, infant diagnosis, antiretroviral initiation and monitoring. WHO 2021, March (https://bit.ly/30P7RtI).
- Nodell B. Lessons from Africa on how to fight an infection. University of Washington Medicine. Newsroom, Postscript 2020, 19 March
(https://bit.ly/3eiwTom). - Mugerwa I, Boova T, Van Der Westhuizen, et al. Demonstrating how to improve operational efficiency in Uganda’s overall pathology service – part 3. Beckman Coulter Life Sciences 2021 (https://bit.ly/32kbMQ3).