Improving the precision of peanut allergy testing
The increasing prevalence of peanut allergy has placed a significant burden on patient waiting times in Australian allergy clinics. We hope that the use of 2-step component testing for whole peanut and Ara h2 improves the accuracy of peanut allergy diagnosis, thereby alleviating this problem. This article discusses the clinical implications for Ara h2 testing.
by Thanh D. Dang and Professor Katrina J. Allen
The current diagnosis of peanut allergy
Food allergy affects 4–6% of the western population, with the number of hospital admissions for food related reactions more than doubling in the last decade. Peanuts and tree nuts account for almost half of all food related incidences of anaphylaxis. The diagnosis of peanut allergy is relatively straightforward when there is an unequivocal history of clinical reaction to peanut ingestion [1]. However, diagnosis can be more complicated in cases where the clinical history is not clearly defined, or in children who have not yet been exposed to a food. Positive results for both the skin prick test (SPT) and the blood test for peanut-specific IgE (ImmunoCAP fluorescence enzyme immunoassay) have high sensitivity and low specificity for diagnosis of peanut allergy. Thus, the positive predictive value (PPV) of these tests (i.e. the likelihood of detecting true peanut allergy) is low. Several reports have documented that the use of 95% PPV threshold values has assisted peanut allergy diagnosis in the clinic [2,3]. Nevertheless, for the large majority of patients who have a positive SPT or ImmunoCAP result below 95% PPV, it remains uncertain whether or not there is clinical allergy, and an oral food challenge (OFC) is required to confirm or exclude a diagnosis of food allergy. Although definitive, the OFC is time consuming, costly, and is associated with a risk of anaphylaxis. Thus, new approaches that allow accurate diagnosis of peanut allergy while reducing the need for an OFC are needed.
Due to the rapid rise in rates of sensitisation to foods, allergy services are overwhelmed and food-challenge tests may be difficult to access. Clinicians faced with the difficult task of having to assess for the presence of food allergy based solely upon a positive SPT or ImmunoCAP must err on the side of caution and accept a diagnosis of ‘possible’ food allergy in these situations. This approach may lead to over-diagnosis of peanut allergy in the community, and a potentially unnecessary burden on the healthcare system. Moreover, an incorrect diagnosis of food allergy unnecessarily imposes allergen avoidance and impaired quality of life on the patient and family.
Component resolved diagnosis
Recent progress in molecular biology and biochemistry has led to recombinant production of individual allergenic proteins for peanut. A total of 11 peanut components (Ara h1–11) have been identified with Ara h1–3 considered to be the major peanut allergens [4]. In recent times, the use of Ara h allergens has been suggested to be a more accurate diagnostic tool for the assessment of peanut allergy. Ara h2-specific immunoglobulin-E (Ara h2-sIgE) has been detected in 90–100% of peanut allergic patients and, although these results have been based on small study cohorts, it has been suggested that the sensitivity and specificity of Ara h2-sIgE testing is higher when compared to the current tests used to diagnose peanut allergy [5–7]. In a study of 29 peanut allergic patients, Nicolaou et al. reported a 93% sensitivity and a 100% specificity for Ara h2-sIgE at level of 0.55 kUA/L (UA = allergen specific unit) [8]. However, this small study was carried out on a patient population selected from the clinic and negative controls were not used.
In this study, we assessed whether Ara h2-sIgE could accurately identify peanut allergic status using subjects recruited into the population-based HealthNuts study, where an OFC was performed in all infants with positive peanut SPT irrespective of wheal size or history of previous reaction [9]. This design allows validation of the ability of Ara h2 to predict peanut allergy in a population-based setting.
Methods
The methods for study recruitment, skin prick testing and oral food challenges used in the Melbourne, Australia, based HealthNuts study have been detailed previously [9]. For an estimated sensitivity or specificity of 95% from Nicolaou’s study Ara h2-sIgE data [5], a sample size of 100 allergic and 100 non-allergic participants randomly selected from the HealthNuts study is sufficient to provide a lower limit of 92% for the corresponding 95% confidence interval. Of the 200 subjects analysed, 100 were peanut allergic, confirmed with a positive SPT and peanut food challenge. The remaining 100 peanut tolerant infants, confirmed by a peanut food challenge, consisted of 58 peanut-sensitised and 42 non-peanut sensitised infants, who were randomly selected as negative controls. Allergen-sIgE was measured with the ImmunoCAP System FEIA (Phadia AB, Uppsala, Sweden). Plasma samples were analysed for IgE to whole peanut and Ara h2.
Samples with Ara h2 IgE <0.35 kUA/L were tested for the presence of allergen specific IgE to the other major peanut allergens Ara h 1, 3, 8 and 9.
Ara h2 testing compared to other tests
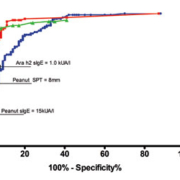
Compared to both skin prick testing and whole peanut sIgE, measurement of Ara h2-sIgE correctly identified more true peanut allergic subjects, when 95% specificity or 95% PPV were applied. If the previously reported 15 kUA/L for peanut sIgE threshold is adopted, which provides the 95% PPV and 98% specificity [Figure 1], the sensitivity of the whole peanut-sIgE test is 26% [95% CI: 18%–36%]. At a comparative specificity of 97%, provided by an Ara h2-sIgE level of 1.00 kUA/L, Ara h2-sIgE testing detects 60% [95% CI: 50%–70%, p<0.001].
How Ara h2 testing can improve peanut allergy diagnoses and outcomes of our study
Figure 2 shows two strategies which incorporate the use of peanut-sIgE or SPT as the first line test followed by Ara h2-sIgE as a second line of testing to help improve the accuracy of distinguishing peanut allergic from peanut tolerant subjects. In the first model, Ara h2 testing of the 95 infants with peanut-sIgE levels between 0.35 and 14.9 kUA/L successfully identified an additional 22 infants as peanut tolerant and 35 infants as peanut allergic [Figure 2a]. Hence incorporating Ara h2-sIgE in combination with peanut-sIgE testing would reduce the number of oral food challenges by almost two-thirds from 95 (47.5%) down to 32 (16%, P<0.001). In the second model, Ara h2 testing of the 50 infants with SPT between 3-8mm identified an additional 6 infants as peanut tolerant and 21 as peanut allergic [Figure 2b], reducing the number of oral food challenges from 50 (25%) to 21 (10.5%, P=0.007).
We found that Ara h2-sIgE testing is more accurate in determining peanut allergy compared to either peanut SPT or whole peanut sIgE alone using the gold standard OFC to confirm presence of true food allergy. Although we demonstrated that the performance of both SPT and Ara h2-sIgE testing to correctly identify peanut allergic and peanut tolerant infants were similar, a SPT is usually performed in a specialist setting. The patient waiting times are at present significant exceeding 18 months in many centres in Australia. By comparison, blood testing for Ara h2 and whole peanut sIgE can be easily be accessed in the community by primary and secondary healthcare professionals with access to diagnostic laboratories. Using our cohort, testing with peanut sIgE followed by Ara h2-sIgE could substantially reduce the number of OFCs required to diagnose peanut allergy by almost two-thirds. Given that the cut-off of ≤0.1 kUA/L for Ara h2 can identify 87% of peanut tolerant infants while only having a 5% false negative rate, this would support the gradual introduction of peanuts into the diet if the child has not already eaten the food. Conversely, a cut-off of 1.00 kUA/L for Ara h2 detects 60% of peanut allergics with a false positive rate of 2%; and can be used to diagnose the presence of peanut allergy. The remainder of subjects that fall between these thresholds would require an OFC to confirm peanut allergy status. While the use of Ara h2 in the diagnosis of peanut allergy in the community has significant advantages, in an allergy clinic setting, peanut SPT still provides a rapid and accurate method for determining peanut allergy, and Ara h2-sIgE could be used as a subsequent test to reduce the number of patients requiring an oral food challenge. One limitation of our results is that our tested population were all 12 month old infants. However, in the absence of data from populations including older age groups, these results are the best available and likely to be reasonably robust for other ages.
Where will Ara h2 testing be applicable?
Ara h2 has been identified as the predominant peanut allergen in a number of countries from Europe, North America, south-east Asia and Australia and may be considered in the diagnosis of peanut allergy [6,8,10–13]. However, the use of Ara h2 sIgE testing may not by applicable to all populations – for example Ara h9 is reported to be the dominant allergen in Spain. In the Australian population, other recombinant allergens tested may not improve detection of peanut allergy amongst subjects with Ara h2-sIgE levels <0.35 kUA/L. In our study, only 9 of the 100 peanut allergic patients had Ara h2-sIgE concentrations <0.35 kUA/L, and only 5 of these 9 subjects had detectable Ara h1, Ara h3, Ara h8, or Ara h9 sIgE levels. Further additional testing of the other peanut allergens on all subjects would be required to identify if there are other allergens that are dominant in our region. Our 2-step testing algorithm ensures that targeted testing of these Ara h allergens can be undertaken in a cost-effective way. That is to say that those children with a positive whole peanut sIgE but negative Arah2 would then require testing for the other Ara h allergens depending on the region of testing and the second most predominant allergen in that region.
Conclusion
Whole peanut IgE followed by Ara h2-sIgE testing should be considered as the preferred 2-step diagnostic tool for determining peanut allergy, as we have shown greater diagnostic accuracy with this method than by either whole peanut sIgE or peanut SPT alone. This will reduce the need for an oral food challenge and ultimately may reduce the strain and demand on clinical allergy services.
References
1. Allen KJ, Hill DJ, Heine RG. Food allergy in childhood. Medical Journal of Australia 2006; 185: 394–400.
2. Sporik R, et al. Specificity of allergen skin testing in predicting positive open food challenges to milk, egg and peanut in children. Clinical and experimental allergy 2000; 30: 1540–1546.
3. Sampson HA. Utility of food–specific IgE concentrations in predicting symptomatic food allergy. The Journal of allergy and clinical immunology 2001; 107: 891–896.
4. Allergen Nomenclature. The Official ‘‘List of Allergens.’’ W.H.O./I.U.I.S. Allergen Nomenclature Sub–Committee. 2010. Available at: http://www.allergen.org. Accessed May 15, 2011.
5. Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component–resolved diagnostics. The Journal of allergy and clinical immunology 2010; 125: 191–197.
6. Knol EF, Knulst AC, Bruijnzeel-Koomen CA, Hoekstra MO, Pasmans SG, Koppelman S, et al. Children with peanut allergy recognize predominantly Ara h2 and Ara h6, which remains stable over time. Clinical and experimental allergy 2007; 37: 1221–1228.
7. Astier C, Morisset M, Roitel O, Codreanu F, Jacquenet S, Franck P, et al. Predictive value of skin prick tests using recombinant allergens for diagnosis of peanut allergy. Journal of Allergy and Clinical Immunology 2006; 118: 250–256.
8. Nicolaou N, Murray C, Belgrave D, Poorafshar M, Simpson A, Custovic A. Quantification of specific IgE to whole peanut extract and peanut components in prediction of peanut allergy. Journal of Allergy and Clinical Immunology 2011; 127: 684–685.
9. Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Thiele L, Tang ML, et al. The HealthNuts population–based study of paediatric food allergy: validity, safety and acceptability. Clinical & Experimental Allergy 2010; 40: 1516–1522.
10. Vereda A, van Hage M, Ahlstedt S, et al. Peanut allergy: Clinical and immunologic differences among patients from 3 different geographic regions. The Journal of allergy and clinical immunology 2011; 127: 603–607.
11. Flinterman AE, Knol EF, Lencer DA, Bardina L, den Hartog Jager CF, Lin J, et al. Peanut epitopes for IgE and IgG4 in peanut–sensitized children in relation to severity of peanut allergy. Journal of Allergy and Clinical Immunology 2008; 121: 737–743.
12. Chiang WC, Pons L, Kidon MI, Liew WK, Goh A, Wesley Burks A. Serological and clinical characteristics of children with peanut sensitization in an Asian community. Pediatric Allergy and Immunology 2010; 21: e429–e38.
13. Codreanu F, Collignon O, Roitel O, Thouvenot B, Sauvage C, Vilain AC et al. A novel immunoassay using recombinant allergens simplifies peanut allergy diagnosis. International Archives of Allergy & Immunology 2011; 154: 216–26.
The authors
Thanh D Dang, BBiomedSc and
Katrina J. Allen*, BMedSc, MBBS, FRACP, PhD
MCRI, Royal Children’s Hospital
Flemington Road, PARKVILLE VIC 3052, Australia
*Corresponding author:
email: katie.allen@rch.org.au