Inductively coupled plasma mass spectrometry: the future of sweat analysis?
Sweat chloride is the gold standard diagnostic test for cystic fibrosis (CF) offering direct measurement of cystic fibrosis transmembrane conductance regulator (CFTR) protein function. Current methods are labour-intensive, complex, time-consuming and require relatively large sample volumes. Inductively coupled plasma mass spectrometry (ICP-MS) is an emerging technology capable of providing rapid and accurate sweat chloride concentrations from small sample volumes.
by Dr Anna Robson, Dr Alexander Lawson and Dr Stephen George
Background
Cystic fibrosis (CF) is a life limiting inherited disorder with an incidence of 1 in 2500–3500 live births in the UK and USA [1]. Mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene translate to dysfunction of the CFTR protein, responsible for controlling transepithelial chloride transport. CF is a multisystem disease, due to the ubiquitous location of the CFTR, however, it is most commonly associated with pulmonary and pancreatic pathologies. Data from the Cystic Fibrosis Foundation Patient Registry (CFFPR) annual report showed that approximately 80% of CF patients are on pancreatic enzyme replacement therapy (PERT) and over 70% of CF mortality is a consequence of respiratory or cardiorespiratory related causes [2].
Implementation of newborn screening pathways for CF has enabled early diagnosis, with advancements in treatment strategies showing significant benefits to patients over the last 25 years. CFFPR data shows that patients now have a median predicted survival of 39.3 years and a higher proportion of adults than children, with CF, was reported in the USA for the first time in 2014 [2]. Patients’ quality of life is also improving with better lung function at the age of 18 years, more patients graduating from higher education and an increased number of viable pregnancies in female patients [2].
There are currently over 2000 known genetic mutations of the CFTR gene associated with CF, many of which can be categorized into five classes [3]. Classes I–III give rise to the most severe phenotypes due to absence or non-function of CFTR, whereas residual CFTR function is observed in patients with class IV–V mutations [2, 3]. Molecular genetic testing has become an increasingly important aid to the diagnostic pathway for CF, especially for patients with rarer mutations and milder forms of the disease. However, molecular analysis is insufficiently sensitive and specific enough to be used as a first line test as rare variants can be missed. Use of next generation sequencing is currently prohibited by cost but may become a more viable option in the future. The UK newborn screening program for CF utilizes a combination of immunoreactive trypsinogen (IRT) and genetic testing for the most common mutations. Although IRT is a good screening tool in neonates, it is not diagnostic and is unsuitable for use in adults. All babies that screen positive for CF are referred for diagnostic confirmation by sweat testing.
Diagnosis of CF
More than six decades since its inception in 1959 [4], quantification of chloride ions in sweat remains the gold standard diagnostic test for diagnosis of CF. In normal functioning sweat glands, isotonic sweat is secreted into the secretory coil. Sodium and chloride are then reabsorbed in the water-impermeable reabsorptive duct via the epithelial sodium channel (ENaC) and the CFTR respectively. Reabsorption of chloride is reduced in CF patients with defective or absent CFTR, thus resulting in sweat electrolyte loss. Sweat concentrations, therefore, provide a direct measurement of electrolyte secretion. Elevated sweat sodium levels are also observed in CF due to the dependence of ENaC activation on CFTR function.
Sweat chloride measurements demonstrate 98% diagnostic specificity for CF [5]. Research has also shown correlations between the type of genetic mutation and chloride concentration [2, 6]. Methods employed in sweat analysis include osmolality, conductivity and electrolyte concentration, however current guidelines developed by the UK Royal College of Paediatrics and Child Health (RCPCH) and the Association of Clinical Biochemistry (ACB) recommend sweat chloride as the analyte of choice for CF diagnosis [7]. Sweat conductivity measurements are accepted for screening purposes in patients over 6 months of age provided that all positive and borderline results are followed up with a chloride measurement [7]. Sweat sodium is no longer recommended for CF diagnosis as it is less reliable than chloride as an indicator of CFTR function [7]. Most laboratories providing sweat analysis measure a combination of analytes, typically chloride with either conductivity or sodium, using the latter two for quality control purposes only [5].
There are currently two accepted methods for collecting sweat following pilocarpine stimulation, the Gibson and Cooke technique (GCT) and the Wescor Macroduct® collection system (WMCS). Using the GCT, sweat is collected onto pre-weighed chloride-free filter paper and eluted in the laboratory for analysis. Sweat is collected into a plastic capillary in the WMCS closed system, thus reducing analytical errors associated with weighing, dilution and evaporation. A minimum sweat secretion rate of 1 g/m2/min is recommended to obtain an accurate chloride concentration. This equates to approximately 75 mg or 15 μL of sweat, depending on the collection method, in 30 minutes [1]. Low sweat rates indicate either suboptimal sweat secretion by the patient or sample evaporation, both of which can affect the accuracy of electrolyte measurements [5]. RCPCH/ACB guidelines therefore recommend duplicate analysis, on each sweat sample collected, to minimize analytical imprecision due to the manual nature of sweat testing [7]. Studies report conflicting data in relation to which collection system yields a higher insufficient rate for sweat analysis [8, 9]. However, the primary limitation of WMCS compared to GCT is reduced sample volume for analysis when a sufficient sweat rate has been achieved. A recent UK audit at Heart of England NHS Foundation Trust (HEFT) showed that more than 30% of samples with a sufficient sweat rate (>15 μL) were insufficient for duplicate ion selective electrode (ISE) analysis when using the WMCS.
Current methods of analysis
Currently accepted methods for sweat chloride quantification include coulometry, colourimetry, and ISE analysis, of which coulometry is the most commonly used (112/161 UK laboratories enrolled in the UKNEQAS external quality assurance (EQA) scheme). Sweat-Chek equipment is recommended for conductivity measurements and accepted methods for sodium include flame photometry, ISE and atomic absorption spectroscopy [6, 7]. All of these methods require manual measurement of each sample, thus occupying the time of a specially trained member of staff for the analysis duration. Dedicated instrumentation is often used for each analyte and sample volume requirements are relatively large compared to the minimum accepted collection using the WMCS. Sweat analysis is therefore complex and time-consuming. The need for dedicated instrumentation, specifically trained staff and laboratory time also carries a cost burden for NHS laboratories with increasing budget restrictions.
Inductively coupled plasma mass spectrometry
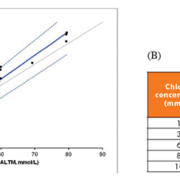
At HEFT, we have developed a method for the analysis of sweat sodium and chloride using inductively coupled plasma mass spectrometry (ICP-MS). ICP-MS is an emerging technology in the clinical laboratory, primarily used for determining the elemental composition of samples and recently applied for sweat analysis [10, 11]. Benefits of ICP-MS include rapid and batched measurements, reproducibility, increased sensitivity and specificity compared to traditional methods, simultaneous quantification of multiple elements and ‘walk-away’ analysis. The ICP-MS method for sweat sodium and chloride uses a simple dilute and shoot approach, requiring just 2 μL of sample. The method was found to be both accurate and precise. Comparison studies using EQA samples showed a 3.6 % bias compared to target values [UKNEQAS EQA scheme all laboratory trimmed mean (ALTM)] (Fig. 1a); however, this was not statistically significant at clinical decision limits (30–60 mmol/L) and results were comparable to the coulometry method currently in use. Recommended acceptable precision (<5% CV), as defined by RCPCH/ACB guidelines, was obtained for all clinically relevant concentrations for quality control (QC) samples (Fig. 1b) [1, 7].
ICP-MS has numerous advantages, compared to coulometry, for sweat chloride analysis. Firstly, the low sample volume requirement allows for duplicate measurements on minimum viable samples (15 μL). Analysis run time is approximately 30–60 minutes depending on the number of samples, and ICP-MS is a ‘walk-away’ method. Staff are, therefore, available to carry out other work once the samples have been prepared and placed on the auto-sampler which is advantageous compared to current methods that can occupy a dedicated member of staff for up to half a day (Fig. 2). Improvements in laboratory efficiency are gained by moving away from dedicated chloride and conductivity meters to analysis using equipment already in use for the trace metal service. The main limitation of chloride analysis by ICP-MS at present is contamination due to the instrument tuning solution containing hydrochloric acid. Hence, care must be taken to ensure that the lines of the inlet system have been rinsed for long enough to remove any residual chloride before analysis. Clearly a tuning solution containing a different acid (e.g. nitric acid) would be beneficial and work is underway to source such a reagent. Overall, ICP-MS provides a much more efficient and cost-effective process for sweat analysis as illustrated in Figure 2.
Summary
Sweat chloride analysis is the gold standard test for diagnosis of CF; however, current methods are time-consuming, costly and require large sample volumes relative to the minimum acceptable collection. ICP-MS is a relatively new analysis platform in the clinical environment and is therefore not yet included in any guidelines. However, this technique presents an attractive alternative to current methods for rapid and accurate sweat analysis using small sample volumes. ICP-MS has the potential to benefit sweat testing, improving efficiency and reducing costs in the clinical laboratory.
Acknowledgements
The authors would like to acknowledge Dr Chris Chaloner PhD FRCPath and Lesley Tetlow FRCPath, Central Manchester University Hospitals NHS Foundation Trust, for their assistance in proofreading the manuscript.
References
1. Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, Durie PR, Legrys VA, Massie J, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008; 153:S4–S14.
2. Cystic Fibrosis Foundation Patient Registry Annual Data Report 2014. (https://www.cff.org/2014-Annual-Data-Report.pdf)
3. Veit G, Avramescu RG, Chiang AN, Houck SA, Cai Z, Peters KW, Hong JS, Pollard HB, Guggino WB, et al. From CFTR biology toward combinatorial pharmacotherapy: expanded classification of cystic fibrosis mutations. Mol Biol Cell. 2016; 27(3): 424–433.
4. Gibson LE, Cooke RE. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Paediatrics 1959; 23(3): 545–549.
5. LeGrys VA. Sweat testing for the diagnosis of cystic fibrosis: Practical considerations. J Pediatr. 1996; 129: 892–897.
6. Mishra A, Greaves R, Massie J. The relevance of sweat testing for the diagnosis of cystic fibrosis in the genomic era. Clin Biochem Rev. 2005; 26(4): 135–153.
7. Royal College of Paediatrics and Child Health. Guidelines for the performance of the sweat test for the investigation of cystic fibrosis in the UK, 2nd version. March 2014. (http://www.rcpch.ac.uk/system/files/protected/page/Sweat%20Guideline%20v3%20reformat_2.pdf)
8. Laguna TA, Lin N, Wang Q, Holme B, McNamara J, Regelmann WE. Comparison of quantitative sweat chloride methods after positive newborn screen for cystic fibrosis. Pediatr Pulmonol. 2012; 47: 736–742.
9. Hammond KB, Turcios NL, Gibson LE. Clinical evaluation of the macroduct sweat collection system and conductivity analyzer in the diagnosis of cystic fibrosis. J Paediatr. 1994; 124(2): 255–260.
10. Pullan NJ, Thurston V, Barber S. Evaluation of an inductively coupled plasma mass spectrometry method for the analysis of sweat chloride and sodium for use in the diagnosis of cystic fibrosis. Ann Clin Biochem. 2013; 50(Pt 3): 267–270.
11. Collie JT, Massie RJ, Jones OA, Morrison PD, Greaves RF. A candidate reference method using ICP-MS for sweat chloride quantification. Clin Chem Lab Med. 2016; 54(4): 561–567.
The authors
Anna Robson*1 PhD; Alexander Lawson2 PhD, FRCPath; Stephen George2 PhD, FRCPath
1Department of Clinical Biochemistry, Central Manchester University Hospitals NHS Foundation Trust, Newborn Screening Laboratory, Genetic Medicine, St. Mary’s Hospital, Oxford Rd, Manchester, UK
2Department of of Clinical Chemistry and Immunology, Birmingham Heartlands Hospital, Bordesley Green East, Birmingham, UK
*Corresponding author
E-mail: anna.robson@cmft.nhs.uk