Insight into high-sensitivity troponin tests for the early rule-out of NSTEMI
In August 2020 new guidance was issued by the National Institut e for Health and Care Excellence on the use of high-sensitivity troponin tests for the early rule-out of non-ST-elevation myocardial infarction (NSTEMI). CLI caught up with Heather Read-Harper from Beckman Coulter to find out more about the use of cardiac troponin biomarkers in the evaluation of patients arriving at the Accident and Emergency department with chest pain.
Many people arrive at Accident and Emergency department (A&E) with chest pain. What are the different diagnoses that can be made?
Chest pain is a very common reason for people to arrive in A&E and it is important to rapidly and accurately identify those with cardiac conditions that require critical management while ensuring those with non-cardiac pain can be safely discharged without hospitalization. Around four out of five of those presenting with chest pain are non-cardiac related, examples include anxiety and gastrointestinal or lung-related conditions. Cardiac-related chest pain can be very severe and involves myocardial ischemia, which denotes diminished perfusion or myocardial infarction (MI), where cell death occurs as a result of this reduced perfusion. This type of cardiac injury encompasses several conditions, such as non-ST-elevation MI (NSTEMI), ST-elevation MI (STEMI) and unstable angina and is generally referred to as acute coronary syndrome (ACS).
How are these diagnoses usually made?
Every A&E department follows a chest pain assessment procedure, which includes identifying risk factors (e.g. history, weight, age, blood pressure) alongside physical examination, such as 12-lead electrocardiogram (ECG) and measurement of cardiac biomarkers.
What is difficult about the diagnosis/ exclusion of NSTEMI and why is early rule-out important?
In some patients with acute chest pain, an ST-segment elevation will be evident on the 12-lead ECG. This is indicative of severe myocardial ischemia and means these patients can be triaged quickly. The majority, however, will not have ST-segment elevation and, in some cases, may even have a completely normal ECG. ECG should not be used alone to rule out an ACS, as NSTEMI is still a possibility. NSTEMI can only be reliably diagnosed with the addition of cardiac biomarker analysis. Guidelines, including The European Society of Cardiology (ESC) 2020 Acute coronary syndromes (ACS) in patients presenting without persistent STsegment elevation (Management of) guidelines [1] and the National Institute for Health and Care Excellence (NICE) High-sensitivity troponin tests for the early rule out of NSTEMI [2] now advocate the use of cardiac biomarkers, specifically high-sensitivity troponin (hsTn), to identify NSTEMI patients quickly to ensure improved outcomes. hsTn also supports rapid rule-out of an NSTEMI diagnosis in patients with very low values, allowing clinicians to move quickly to consideration of differential diagnoses, and may free up additional resources that can be refocused on patients with higher acuity conditions.
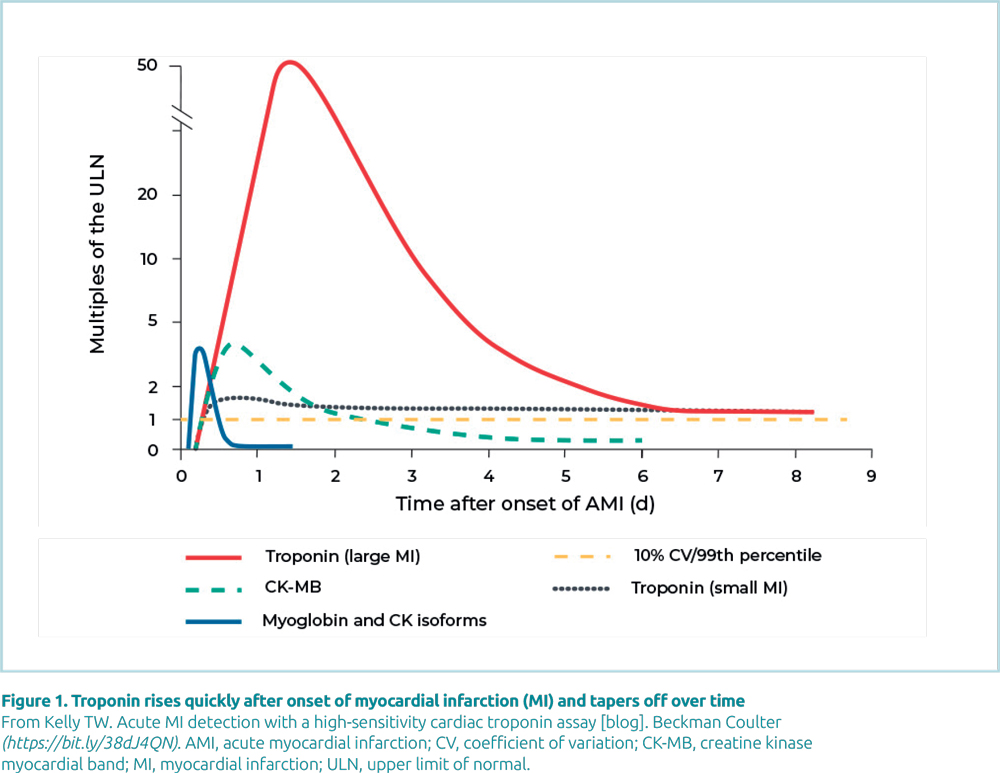
In MI, cardiac troponin (cTn) levels rise in the hours after the onset of cardiac symptoms, reaching a peak at 12–16 hours and can remain elevated for 4–9 days after MI. There are, however, numerous pathologies that can potentially cause Tn elevations without overt ischemic heart disease. These pathologies include, but are not limited to, congestive heart failure, hypertension, hypotension, arrhythmias, pulmonary embolism, severe asthma, sepsis, critical illness, myocarditis, stroke, drug toxicity, end stage renal disease and rhabdomyolysis with cardiac injury. Importantly, these other etiologies rarely demonstrate the classic rising and falling pattern experienced with a MI. As a result, serial testing is often required when the clinical scenario is unclear.
What cardiac biomarkers are available for analysis and why are they useful?
cTn emerged over 15 years ago and because it is the most specific marker for cardiac muscle, it has become the biomarker of choice for confirming cardiac muscle damage (Fig. 1). Myoglobin and CK-MB (creatine kinase myocardial band) were traditionally used for this purpose, but myoglobin is released with damage to any kind of muscle and, while CK-MB is more specific for cardiac muscle, it is present in other forms of muscle.
The performance of cTn assays has changed significantly with recent improvements to sensitivity and precision. Guidelines reinforce the importance of using the 99th percentile upper reference limit (URL) of healthy individuals as the cut-off to aid in diagnosis of MI. The use of the 99th percentile URL cut-offs has increased the number of patients that are monitored for MI and helped identify patients with myocardial injury and elevated cTn due to other conditions.
Current direction from the International Federation of Clinical Chemistry (IFCC) states high-sensitivity assays must have analytical imprecision <10% CV at the 99th percentile URL of a healthy population. This level of precision ensures repeatability of results and lowers the chance of patient misclassification. In addition, the IFCC states that a high-sensitivity assay must be able to measure cTn above the limit of detection in >50% of a healthy population.\
In patients presenting in A&E, only 3.5% are ultimately diagnosed with acute MI, but over 50% of patients are admitted for observation or as inpatients. Identification of a significant change in cTn values is one critical element in confirming a diagnosis of AMI for patients under observation. As a result, cTn testing algorithms cited in ESC and NICE guidance also indicate that diagnosis of AMI requires observation of a rise and/or fall of cTn values with at least one value above the 99th percentile of URL together with at least one of the following: symptoms of acute myocardial ischemia; new ischemic ECG changes; development of pathological Q waves; imaging evidence of new loss of viable myocardium or new regional wall motion abnormality in a pattern consistent with ischemic etiology; or identification of coronary thrombus by angiography, including intracoronary imaging or autopsy. This change in cTn levels is typically measured between two samples taken at 1- to 3-hour intervals depending on the pathway used in the hospital. It is important that a Tn assay has excellent precision to be able to measure this change. Measuring a significant delta value between samples also increases the assay’s specificity. Improved specificity ensures that acute ischemic events and chronic cardiac disease without overt ischemic heart disease are differentiated and the patient is triaged quickly and appropriately.
The use of sex-specific 99th percentile URL reference ranges for high-sensitivity cTn assays is another addition to recent guideline updates. Women often present with symptoms and ECG abnormalities that are not typically associated with AMI, complicating diagnosis. Women, on average, have less myocardial mass than men. Accordingly, any ischemic event damages a smaller absolute quantity of myocardium, which may lead to lower levels of circulating cTn. For this reason, sex-specific reference intervals may improve the diagnostic accuracy of high-sensitivity assays for women.
Combining a hsTn assay with well-defined patient management pathways, quickly indicates if a person is having a MI or not. This leads to reduced waiting times and anxiety for the patient, while also allowing hospitals to deliver safe and high quality urgent and emergency care.
What is the NICE guidance on the use of these biomarker tests for early rule-out of NSTEMI and what has changed with the latest update?
The NICE diagnostic guidance makes evidence-based recommendations on new diagnostic technologies for adoption in the NHS.
High-sensitivity troponin tests for the early rule out of NSTEMI [DG40] [2] was published in August 2020 to ensure that guidance is based on current evidence for the management of NSTEMI and includes new recommendations for high-sensitivity tests developed since the last publication guidance document published in 2014. In addition, it provides detailed recommendations on how to use hsTn assays in a clinical setting.
The new guidance has expanded the range of recommended hsTn assays, including the Access hsTnI (Beckman Coulter; Fig. 2), for the early rule-out of NSTEMI in people presenting to A&E with chest pain and suspected ACS.
Recommended testing strategies for 0/1 hour and 0/2 hour algorithms are described as clinically and cost-effective strategies for rapid rule-out of MI.
What do you envisage for the future development of MI diagnosis/exclusion?
There will always be opportunities for improvement as technology evolves. Ongoing research in a diversity of areas that show potential to expand the clinical utility of hsTn assays in the future. For instance, hsTnI assays have demonstrated the ability to risk-stratify patients with stable cardiovascular disease and identify those at higher risk for future adverse events. More research, and ultimately interventional clinical trials will be needed to determine the true benefit of using hsTnI in expanded indications for MI diagnosis, but there is certainly a lot of untapped potential.
The interviewee
Heather Read-Harper BSc, Senior European Marketing
Manager for Immunoassay and Clinical Chemistry
Beckman Coulter United Kingdom Limited, High Wycombe, Buckinghamshire, UK
For more information visit Beckman Coulter Access hsTnI
(https://www.beckmancoulter.com/products/immunoassay/access-hstni)
References
- 2020 Acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation (management of) guidelines. European Society of Cardiology 2020 (https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/ Acute-Coronary-Syndromes-ACS-in-patients-presentingwithout- persistent-ST-segm).
- High-sensitivity troponin tests for the early rule out of NSTEMI. Diagnostics guidance [DG40] (https://www.nice.org. uk/guidance/dg40).