Interference in thyroid function tests – problems and solutions
Interference in immunoassay is a well described phenomenon and all clinical immunoassays, including thyroid function tests, are potentially at risk. Spurious results can lead to over investigation or mismanagement if not detected, but a proactive approach by the laboratory will help to identify and resolve these problems.
by Dr Olivia Bacon and Dr David J. Halsall
Background
Thyroid disorders are relatively common, and are associated with long-term morbidity and mortality. Clinical signs and symptoms are often non-specific, so reliable laboratory tests are critical for diagnosis. Therefore, thyroid function tests (TFTs) are frequently requested immunoassays with around 10 million results being reported each year by UK laboratories. In the UK, TFTs typically include a high sensitivity immunoassay for thyroid stimulating hormone (TSH) with an immunoassay estimation of non-protein bound thyroxine (fT4), either run simultaneously or added if the TSH value is outside the reference interval [1].
For the majority of tests, both results will be within the reference interval and thyroid disease can be excluded. In some patients TFTs support the diagnosis of hypothyroidism (raised TSH with fT4 low, or lownormal) or hyperthyroidism (TSH undetectable, and fT4 elevated), and these results will confirm clinical findings. However, due to the high volume of TFTs performed, it is not unusual for the laboratorian to be faced with a set of TFTs that are either internally inconsistent, or incompatible with the clinical details provided. Many medications can affect the thyroid axis, as can other non-thyroidal pathologies; these are often transient, but can cause unusual patterns of TFT. Much rarer genetic or pituitary conditions can also cause discordant TFTs [2]. However, if drug effects are excluded, it is necessary at this stage for the laboratorian to consider that one of the TFT results is incorrect, as analytic error is at least as common as these rare thyroid conditions. As spurious TFT results can lead to over investigation, or even inappropriate treatment, it is critical, but not trivial, for the laboratory to confirm the analytical validity of the TFT results.
In one study of more than 5000 samples received for TSH analysis, assay interference with the potential to adversely affect clinical care was detected in approximately 0.5% of patients [3]. This equates to a rather alarming 50,000 tests per annum in the UK.
Although assay design is continually improving, no routine immunoassay is currently robust to interference. Technical errors with many routine chemistry methods caused by inappropriate sample collection or handling, chemical or spectral interference can be detected during result validation. However, detection of spurious TFT immunoassay results is more challenging as there is no automatic ‘flag’ from the analyser, and there is usually a wide range of plausible values for these analytes, making it difficult to question those which are ‘suspicious’. Consequently clinical validation, where results are checked for discordance with the clinical correlates and other laboratory tests, is used to detect potentially incorrect results before reporting. For TFTs this is aided by the characteristic reciprocal relationship between TSH and fT4 in patients with an intact pituitary–thyroid axis.
Mechanisms of interference in TSH assays
Endogenous interfering antibodies are a well described cause of immunoassay interference [4]. In TSH assays these antibodies can have affinity for TSH itself or towards assay components. Anti-reagent antibodies can be ‘anti-animal’ antibodies, specific to the species in which the reagent antibody was raised, or weak, polyspecific ‘heterophilic’ antibodies, which may be part of the natural process of the generation of antibody diversity [5]. Anti-animal antibodies are more prevalent in animal handlers or patients treated with therapeutics based on animal immunoglobulins.
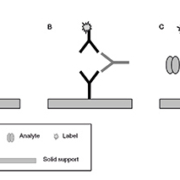
Anti-reagent antibodies can interact with either the capture or detection antibodies in two-site assays, blocking the generation of signal in the presence of analyte (false negative result) or by causing antibody cross-linking in the absence of analyte (false positive result) [Fig. 1].
Anti-TSH antibodies can generate high molecular weight TSH : antibody complexes (‘macro-TSH’). Depending on the exact site of the antibody–analyte interaction, false positive TSH results may occur as the macro-TSH is unlikely to be biologically active [6].
Detection of interference in TSH assays
Once suspected, a robust laboratory strategy is required for confirming or excluding assay interference. Method comparison using an alternative method is often used as the first step. Most laboratories use two-site immunoassays for TSH, but assay formulations, antibody species and incubation times vary between manufacturers. Varying amounts of blocking agents, designed to prevent non-specific binding of heterophile antibodies, may be included. Significant disagreement between two TSH methods is a strong indicator of assay interference.
Dilution studies are a simple but effective tool to investigate the analytical validity of an immunoassay. Non-linearity to dilution suggests a result is unreliable. However, although a good ‘rule in’ test, linearity to dilution alone cannot be used to exclude interference [3,7].
Immunosubtraction is a useful method to confirm the presence of antibody interference. This can be done crudely using polyethylene glycol (PEG) precipitation or more specifically using anti-immunoglobulin agaroses. Proprietary heterophile blocking tubes can also be used to confirm the presence of this class of interferent [3,4].
Once assay interference is established it can still be difficult to determine the correct TSH value, as there is no ‘gold standard’ method for TSH. However, an alternative immunoassay result which gives the expected responses to dilution and immunosubtraction, and correlates with fT4 results plus clinical findings, can be used with a reasonable degree of confidence.
Mechanisms of interference in fT4 assays
fT4 assays present a particular analytical challenge as >99.9% of T4 in the serum is protein bound, and the unbound T4 fraction must be measured without upsetting the equilibrium between the two fractions [8]. Therefore, an abnormal T4 binding protein, or agent which affects binding protein affinity in vitro, has the potential to generate incorrect results. Most commercial fT4 assays are one-site immunoassays based on competitive principles, using either labelled T4 analogue or anti-T4 antibodies for detection. Both heterophile and anti-T4 antibodies therefore also have the potential to interfere with these methods [4].
Non-esterified fatty acids (NEFAs) are a common T4 displacing agent as they can release T4 from the low affinity, high capacity T4 binding site on albumin. NEFAs can be generated in vitro, usually as a consequence of heparin therapy, which stimulates the action of lipoprotein lipase on triglyceride. Although the measured fT4 result is genuinely high, it does not reflect the in vivo situation [9].
Familial dysalbuminaemic hyperthyroxinaemia (FDH) is a benign genetic condition where the affinity of albumin for T4 is increased, such that circulating albumin-bound T4 is elevated. Despite the high total T4 (tT4), concentrations of free hormone in vivo are unaffected due to the homeostatic regulation of the thyroid axis. However, FDH is often associated with falsely high fT4 measurements using commercial immunoassays [10] [Fig. 2]. Both the increased affinity of the variant albumin for some labelled T4 analogues, as well as potential disruption of the T4 : albumin equilibrium during the assay, are likely mechanisms. The presence of the FDH mutation can be confirmed using molecular genetic approaches.
Detecting interference in fT4 assays
Despite the greater analytical challenge, confirming interference in fT4 assays can be easier than for TSH due to the availability of physical separation methods, such as equilibrium dialysis, as ‘gold standard’ assays [8]. However, these methods are technically difficult and not available in most clinical biochemistry laboratories. Also, these methods do not solve the in vitro problems of hormone displacement.
Again a first approach is often method comparison, using a different immunoassay architecture. Dilution and immunosubtraction studies can also be informative, although some fT4 methods are not robust to matrix effects so careful control experiments are required.
Measurement of total rather than free T4 can be useful in situations where there is a suspicion of abnormal T4 binding proteins. For example, total T4 will be elevated in the presence of anti-T4 antibodies and in FDH.
Clinical causes of aberrant TFTs
As mentioned above there are well described pharmacological and pathological causes of unusual TFTs; an increased awareness of analytical artefacts should not detract from the detection of these conditions. For example thyroxine treatment, a TSH secreting pituitary tumour (TSHoma), the genetic condition thyroid hormone resistance, FDH or TFT antibody interference can give elevated fT4 results with a TSH within the reference interval. Attempts by the laboratory to exclude assay interference should complement both the diagnosis of transient and genetic thyroid conditions as well as the more common drug related effects.
Conclusions and future directions
Immunoassay manufacturers have invested considerable resources into reducing the potential for antibody-mediated assay interference, for example by including blocking agents, or using antibody fragments rather than intact antibodies as assay reagents. Although these measures are effective, it is worth bearing in mind that changes to assay formulations may introduce novel types of interference. We have observed negative interference in one fT4 assay which appears related to the presence of a blocking agent introduced to reduce the risk of positive interference in this method [11]. Mass spectrometric methods have been introduced to eliminate antibody interference in both fT4 and tT4 methods, but unfortunately the fT4 methods still require careful optimization to avoid interference caused by binding proteins and displacing agents.
As current TFT methods remain prone to analytical interference the clinical laboratory must remain vigilant to the potential for assay interference, promote effective communication with requesting clinicians, and have procedures in place for investigation of discordant results.
References
1. Association for Clinical Biochemistry (ACB), British Thyroid Association (BTA), British Thyroid Foundation (BTF). UK guidelines for the use of thyroid function tests.2006; www.acb.org.uk/docs/TFTguidelinefinal.pdf.
2. Gurnell M, Halsall DJ, Chatterjee VK. What should be done when thyroid function tests do not make sense? Clin Endocrinol. (Oxf) 2011; 74(6): 673–678.
3. Ismail AA, Walker PL, Barth JH, Lewandowski KC, Jones R, Burr WA. Wrong biochemistry results: two case reports and observational study in 5310 patients on potentially misleading thyroid-stimulating hormone and gonadotropin immunoassay results. Clin Chem. 2002; 48(11): 2023–2029.
4. Despres N, Grant AM. Antibody interference in thyroid assays: a potential for clinical misinformation. Clin Chem. 1998; 44: 440–454.
5. Kaplan IV, Levinson SS. When is a heterophile antibody not a heterophile antibody? When it is an antibody against a specific immunogen. Clin Chem. 1999; 45: 616–618.
6. Halsall DJ, Fahie-Wilson MN, Hall SK, Barker P, Anderson J, Gama R, Chatterjee VK. Macro thyrotropin-IgG complex causes factitious increases in thyroid-stimulating hormone screening tests in a neonate and mother. Clin Chem. 2006; 52: 1968–1969.
7. Ross HA, Menheere PP, Thomas CM, Mudde AH, Kouwenberg M, Wolffenbuttel BH. Interference from heterophilic antibodies in seven current TSH assays. Ann Clin Biochem. 2008; 45: 616.
8. Thienpont LM, Van Uytfanghe K, Poppe K, Velkeniers B. Determination of free thyroid hormones. Best Pract Res Clin Endocrinol Metab. 2013; in press.
9. Stockigt JR, Lim CF. Medications that distort in vitro tests of thyroid function, with particular reference to estimates of serum free thyroxine. Best Pract Res Clin Endocrinol Metab. 2009; 23(6): 753–767.
10. Cartwright D, O’Shea P, Rajanayagam O, Agostini M, Barker P, Moran C, Macchia E, Pinchera A, John R, Agha A, Ross HA, Chatterjee VK, Halsall DJ. Familial dysalbuminemic hyperthyroxinemia: a persistent diagnostic challenge. Clin Chem. 2009; 55(5): 1044–1046.
11. Bacon O, Gillespie S, Koulouri O, Bradbury S, O’Toole A, Stuart-Thompson D, Taylor K, Pearce S, Gurnell M, Halsall DJ. A patient with multiple Roche serum immunoassay interferences including false negative serum fT4. Ann Clin Biochem. 2013; 50(Suppl 1): T50.
The authors
Olivia Bacon PhD and David Halsall* PhD, FRCPath, CSci
Department of Clinical Biochemistry and Immunology, Addenbrooke’s Hospital, Cambridge, UK
*Corresponding author
E-mail: djh44@cam.ac.uk