Interpreting hematology results in pediatrics – a moving target?
Accurate interpretation of laboratory tests is essential to diagnosis and monitoring of many pediatric disorders. Hematology test results in children cannot be interpreted using adult reference intervals owing to the vast changes that occur throughout childhood from the neonatal period to adolescence. Consequently, the Canadian Laboratory Initiative on Pediatric Reference Intervals (CALIPER) programme has recently established both discrete and continuous pediatric reference intervals for hematology parameters based on testing of hundreds of healthy children and adolescents. These comprehensive pediatric reference intervals enable more accurate test result interpretation and improved clinical decision-making in children and adolescents.
Background
Hematology testing is a key component of health assessment in children. As one of the most commonly ordered test panels, the complete blood count (CBC) plays an integral role in the diagnosis and prognostication of various pediatric pathologies, including anemia, infection, and malignancy. Appropriate identification of hematological abnormalities in children is critical to prevent unnecessary complications and prompt appropriate follow-up and treatment.
Blood test results are typically interpreted using reference intervals (RIs). RIs represent a range of values (2.5th to 97.5th percentiles) that can be considered ‘healthy’ with test results above or below flagged as abnormal. Establishing these healthassociated benchmarks in childhood and adolescence is challenging as several covariates, such as age, sex and ethnicity, must be considered [1,2]. Indeed, normal pediatric growth and development significantly influences hematology markers. For example, a hypoxic environment in utero initially causes increased hemoglobin and reticulocytes, which quickly start to fall as a result of decreased red blood cell (RBC) production during the first 6–8 weeks of life, decreased RBC lifespan, and increased blood volume. Immune adaptations and changes in growth factor production also occur in the first few months of life. As adolescence approaches, the onset of puberty drives differential production of androgens and estrogens with direct impact on erythropoiesis. RIs derived in adult populations do not capture these pediatric physiological trends; therefore, the use of adult RIs for interpretation of hematology results in children is inappropriate and significantly increases the risk of diagnostic error.
Canadian Laboratory Initiative on Pediatric Reference Intervals (CALIPER)
The CALIPER project aims to improve the interpretation of blood tests in children by establishing a comprehensive database of age- and sex-specific RIs for important biomarkers of health and disease (www.caliperdatabase.org). To date, CALIPER has recruited over 12 300 healthy Canadian children and adolescents, establishing accurate and robust RIs for over 200 biomarkers on common analytical platforms [2]. Initial studies focused on biochemical analytes, including enzymes, proteins, antibodies, hormones and other important clinical laboratory tests [2]. These data are used by thousands of institutions worldwide and have directly benefitted pediatric clinical laboratories by serving as an up-to-date resource for blood test interpretation from birth to 18 years. Additional international initiatives have also contributed to addressing gaps in the availability of pediatric RIs for important biomarkers, including HAPPI Kids (Australia), PRINCE Study (China), PEDREF (Germany), and ChildX (USA) [2]. Very few initiatives have studied hemato-logical indices in healthy children and adolescents on benchtop and analytical platforms, including Beckman Coulter HmX/GenST [3–5], Siemens Advia [6,7] and Abbott Cell-Dyn [8]. Available studies are limited as they do not include neonates/infants and/or do not report findings on all CBC components, such as the white blood cell (WBC) differential [3–8]. This is likely to be because of various challenges, including the need to run samples shortly after collection as well as requirements for a large sample size to account for expected physiological variation across the pediatric age range. Taken together, this critical evidence gap compromises the ability of clinicians to interpret hematology test results in children and make informed follow-up and treatment decisions.
Closing the gaps – CALIPER hematology studies
In 2020, CALIPER aimed to address this gap by establishing comprehensive pediatric RIs for hematology parameters across several analytical platforms in a large representative cohort of healthy Canadian children and adolescents for the first time [9–11]. This resulted in three publications (by Higgins et al. [9], Tahmasebi et al. [10], and Bohn et al. [11]) establishing comprehensive pediatric RIs for several hematology parameters on the Beckman DxH520 (27 hematology parameters), Beckman DxH90 (47 hematology parameters), and Sysmex XN-3000 (25 hematology parameters) systems, respectively. To summarize, approximately 600 healthy children and adolescents were recruited via community initiatives at schools and other centres with informed consent. Participation required completion of a health questionnaire and blood donation. Whole blood was collected in K2EDTA tubes and analysed within 8 hours of collection on three instruments at The Hospital for Sick Children in Toronto, Canada (i.e. Beckman DxH 900, Beckman DxH 520, Sysmex XN-3000) [9–11]. Participants were excluded if they were pregnant, had history of chronic/acute illness within the previous week, and/or reported regular use of prescribed medications. Additional samples were collected from apparently healthy and metabolically stable infants, children, and adolescents from outpatient clinics at The Hospital for Sick Children. RIs were then established based on Clinical and Laboratory Standards Institute (CLSI) EP28-A3c guidelines [2].
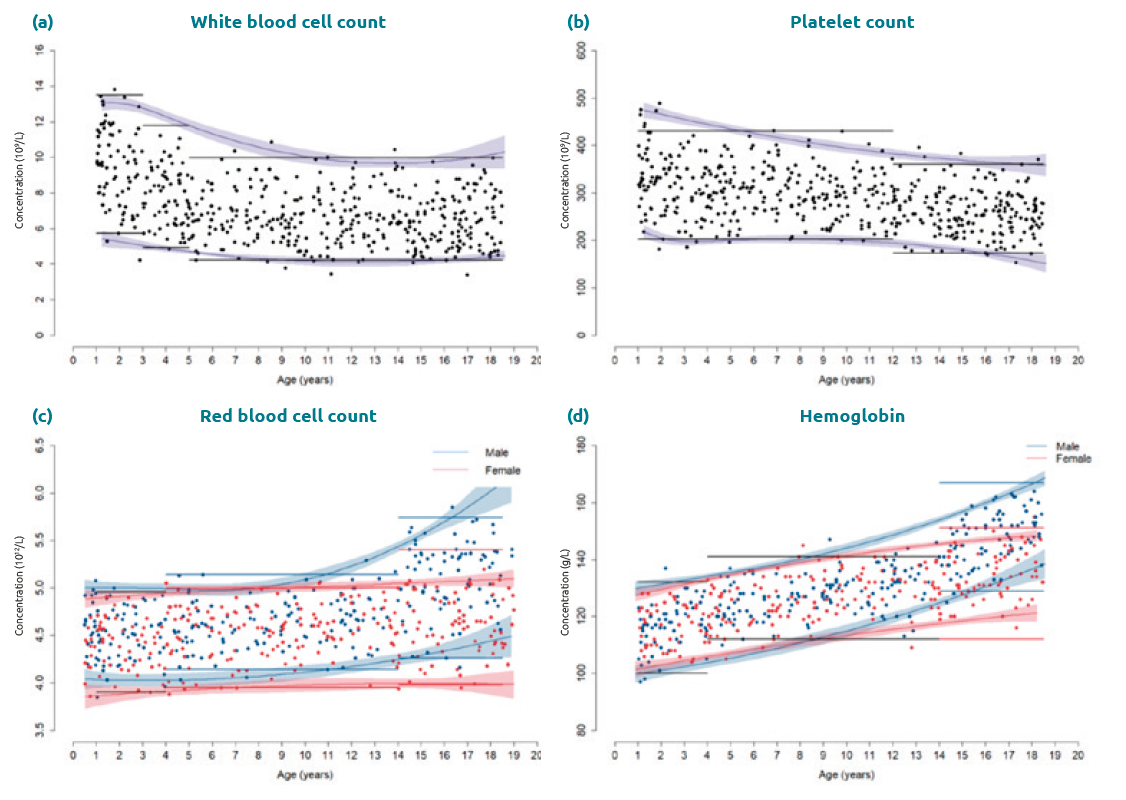
Study findings across instruments emphasized the importance of considering age and sex for evidence-based hematology test interpretation in children and adolescents as well as the analytical reproducibility of indices across platforms. Of the 25 key parameters assessed, 19 required age-partitioning and seven required sexpartitioning (i.e. RBC count, hemoglobin, hematocrit, mean corpuscular volume, RBC distribution width (RDW)-SD, RDW-CV, and monocyte percentage) [9–11]. Novel research parameters, including monocyte distribution width, were also evaluated and demonstrated marked changes with age, requiring age-stratification for test interpretation. Unsurprisingly, statistically significant sex-specific differences were observed for RBCs in puberty with females demonstrating lower RBC counts as well as hemoglobin and hematocrit levels relative to males (Fig. 1). This is likely to be due to testosterone-stimulated erythropoiesis and rapid metabolic demand in males, in addition to menstrual loss in females. In contrast, WBC counts decreased gradually from birth to adolescence [9–11], potentially due to immune adaptations in early life and the development of the adaptive immune system throughout childhood (Fig. 1). The six-part WBC differential also varied based on age, particularly monocyte count, lymphocyte count, and neutrophil count. Further, drastic concentration changes were present in early life for many hematology parameters, reflecting the fetal-to-adult erythropoiesis transition [9–11]. These novel data highlight the dynamic hematological profile observed in healthy children and adolescents, necessitating RI stratification by age and sex for test interpretation. This rich resource is the first to report and capture marked physiological trends in hematological parameters in healthy children and adolescents.
From binning to continuous RIs
In clinical practice and most studies, RIs are stratified into age or sex bins/partitions based on statistical and/or clinical rationale. However, as previously described, studies by CALIPER and others have demonstrated the dramatic changes in hematological indices across the pediatric age range, often requiring an unattainable sample size to develop discrete RIs that accurately reflect concentration dynamics [3–11]. The traditional partitioning approach recommended by CLSI EP28-A3c guidelines may lead to misclas-sification of patient test results in this setting, particularly at the margins of the age groups. Continuous percentile charts have been used for decades to interpret height and weight measurements in children. Recently, there is interest in applying this strategy to laboratory test interpretation as continuous curves may better represent age-specific variation with anticipated clinical value. To expand the utility of CALIPER’s hematology RIs, additional data analysis was completed to establish continuous reference curves for 19 key hematological parameters in healthy children and adolescents for the first time [12].
Non-parametric quantile regression was selected for continuous reference curve establishment [12]. This methodology had been previously established by Asgari and colleagues and applied to CALIPER data for biochemical parameters, including enzymes, proteins and hormones [13]. In contrast to other statistical methods, it is more robust to outliers, variance, and asymmetry [12]. It is important to note that continuous reference curves were only established from 1 to 18.5 years of age, as a result of the extreme variation and limited number of samples in early life. To evaluate performance, flagging rates were compared between partitioned and continuous RIs [12]. Our initial findings when applying this technique to hematology data suggested continuous RIs more accurately estimated reference limits over the pediatric age range (Fig. 1). This was particularly true for markers that change significantly throughout growth and development, including WBC and RBC counts (Fig. 1). Continuous RIs did not present advantages for other parameters with more modest concentration changes with age, such as platelet count (Fig. 1).
If implemented, the ability of continuous reference curves to more accurately represent changes in hematological indices with age in pediatrics may lead to improved clinical decision-making from birth to adolescence. One anticipated challenge is that current laboratory information system frameworks do not present the capability to flag test results based on continuous modelling. However, as more research and resources are dedicated to creating electronic tools to assist clinicians in diagnostic, prognostic, and treatment decisions, continuous RI implementation for select blood tests in pediatrics should become more feasible and made a priority for laboratories.
Concluding remarks
To conclude, children are not small adults. We have observed dynamic changes occurring throughout growth and develop-ment and their subsequent impact on hematology parameters in children and adolescents. It is clear that age- and sex-specific test result interpretation is needed for most hematology parameters, with some benefitting from a continuous RI approach.
Figure 1. Comparison of continuous reference intervals and partitioned reference intervals (a) White blood cell count; (b) platelet count; (c) red blood cell count; (d) hemoglobin using a CALIPER cohort of healthy children and adolescents aged 1–18.5 years. For parameters with no sex-specific difference (a, b), the shaded area (purple) indicates the 95% confidence interval for the continuous reference interval. Partitioned reference intervals for both sexes are shown in black. For parameters with significant sex-specific differences (c, d), the data are colour-coded by sex: reference values, continuous reference intervals and partitioned reference intervals are shown in red (females) and in blue (males).
The authors
Mary Kathryn Bohn1 BSc, Victoria Higgins2 PhD and Khosrow Adeli1* PhD, FCACB, DABCC, FACB
1CALIPER Project, Department of Paediatric Laboratory Medicine, The Hospital for Sick Children, Toronto, ON, Canada
2DynaLIFE Medical Labs, Edmonton, Alberta, Canada
*Corresponding author
E-mail: khosrow.adeli@sickkids.ca
References
- Lyle AN, Pokuah F, Dietzen DJ et al. Current state of pediatric reference intervals and the importance of correctly describing the biochemistry of child development: a review. JAMA Pediatr 2022; doi: 10.1001/jamapediatrics.2022.0794 (Epub ahead of print).
- Adeli K, Higgins V, Trajcevski K, White-Al Habeeb N. The Canadian laboratory initiative on pediatric reference intervals: A CALIPER white paper. Crit Rev Clin Lab Sci 2017;54(6):358–413
(https://doi.org/10.1080/10408363.2017.1379945). - Taylor MRH, Holland CV, Spencer R et al. Haematological reference ranges for schoolchildren. Clin Lab Haematol 1997;19(1):1–15 (https://onlinelibrary.wiley.com/doi/epdf/10.1046/j.1365-2257.
1997.00204.x). - Romeo J, Wärnberg J, Gómez-Martínez S et al. Haematological reference values in Spanish adolescents: the AVENA study. Eur J Haematol 2009;83(6):586–594 (https://doi.org/10.1111/j.1600-0609.2009.01326.x).
- Adeli K, Raizman JE, Chen Y et al. Complex biological profile of hematologic markers across pediatric, adult, and geriatric ages: establishment of robust pediatric and adult reference intervals
on the basis of the Canadian health measures survey. Clin Chem 2015;61(8):1075–1086
(https://doi.org/10.1373/clinchem.2015.240531). - Aldrimer M, Ridefelt P, Rödöö P et al. Population-based pediatric reference intervals for hematology, iron and transferrin. Scand J Clin Lab Invest 2013;73(3):253–261.
- Giacomini A, Legovini P, Gessoni G et al. Platelet count and parameters determined by the Bayer ADVIATM 120 in reference subjects and patients. Clin Lab Haematol 2001;23(3):181–186 (https://doi.org/10.1046/j.1365-2257.2001.00391.x).
- Alnor AB, Vinholt PJ. Paediatric reference intervals are hetero-geneous and differ considerably in the classification of healthy paediatric blood samples. Eur J Pediatr 2019;178(7):963–971.
- Higgins V, Tahmasebi H, Bohn MK et al. CALIPER hematology reference standards (II): improving laboratory test interpretationin children (Beckman Coulter DxH 520–Physician Office Hematology System) with analytical comparison to the Beckman Coulter DxH 900. Am J Clin Pathol 2020;154(3):342–352 (https://doi.org/10.1093/ajcp/aqaa057).
- H, Higgins V, Kathryn Bohn M et al. CALIPER hematology reference standards (I): improving laboratory test interpretation in children (Beckman Coulter DxH 900–Core Laboratory Hematology System). Am J Clin Pathol 2020;154(3):330–341 (https://doi.org/10.1093/ajcp/aqaa059).
- Bohn MK, Higgins V, Tahmasebi H et al. Complex biological patterns of hematology parameters in childhood necessitating age- and sex-specific reference intervals for evidence-based clinical interpretation. Int J Lab Hematol 2020;42(6):750–760 (https://doi.org/10.1111/ijlh.13306).
- Wilson S, Bohn MK, Hall A et al. Continuous reference curves for common hematology markers in the CALIPER cohort of healthy children and adolescents on the Sysmex XN-3000 system. Int J Lab Hematol 2021;43(6):1394–1402 (https://doi.org/10.1111/ijlh.13670).
- Asgari S, Higgins V, McCudden C, Adeli K. Continuous reference intervals for 38 biochemical markers in healthy children and adolescents: comparisons to traditionally partitioned reference intervals. Clin Biochem 2019;73:82–89 (https://doi.org/10.1016/j.clinbiochem.2019.08.010).