Investigation of SARS-CoV-2-specific immune responses
A thorough understanding of the immune response to SARS-CoV-2 is necessary for the evaluation of the efficacy of vaccines and vaccine candidates against this virus. This article discusses how some parts of the humoral and cellular immune responses can be analysed to improve our understanding of how the body reacts to this virus.
Analysis of both humoral and cellular immune responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is critical for understanding how the immune system reacts following infection or vaccination. Key analyses include the quantitative measurement of IgG antibodies against the spike protein S1 domain, which contains the receptor binding domain (RBD), and determination of the neutralizing effect of anti-S1/RBD antibodies. Determination of specific T-cell responses complements the antibody investigation and provides additional evidence of SARS-CoV-2 immunity, especially in patients who do not display detectable levels of antibodies. Comprehensive analysis of SARS-CoV-2 immune responses is particularly important for evaluating the efficacy of vaccines and vaccine candidates. A trio of enzyme-linked immunosorbent assay (ELISA)-based tests (EUROIMMUN) for quantitative measurement of IgG antibodies against S1/RBD, detection of neutralizing antibodies targeting S1/RBD, and determination of SARS-CoV-2-reactive T-cells provides an all-round analysis of SARS-CoV-2-specific immune responses.application of antibody-based therapeutics such as monoclonal antibodies and convalescent plasma.
Quantification of anti-S1/RBD antibodies
Antibodies of class IgG against S1/RBD of the SARS-CoV-2 spike protein play a key role in virus neutralization and building sustained immunity. The RBD of the S1 subunit is responsible for binding to the human cellular receptor angiotensin-converting enzyme (ACE2) and mediating entry of the virus into the host cells. Specific antibodies from previous infection or vaccination can block the RBD and thus prevent the virus from accessing the host cells. It is currently not known what concentration of antibodies confers protection against COVID-19 or how long the immune protection lasts. Establishing the threshold at which immunity can be assumed requires long-term, large-scale studies of infected and vaccinated individuals. Quantitative measurement of anti-S1/RBD antibodies is a valuable tool in the determination of the correlate of protection. In particular, it facilitates assessment of individual immune responses after infection, as well as evaluation of the level of immune reaction achieved through inoculation with spike-protein-based vaccines. Quantitative antibody measurements also support the application of antibody-based therapeutics such as monoclonal antibodies and convalescent plasma.
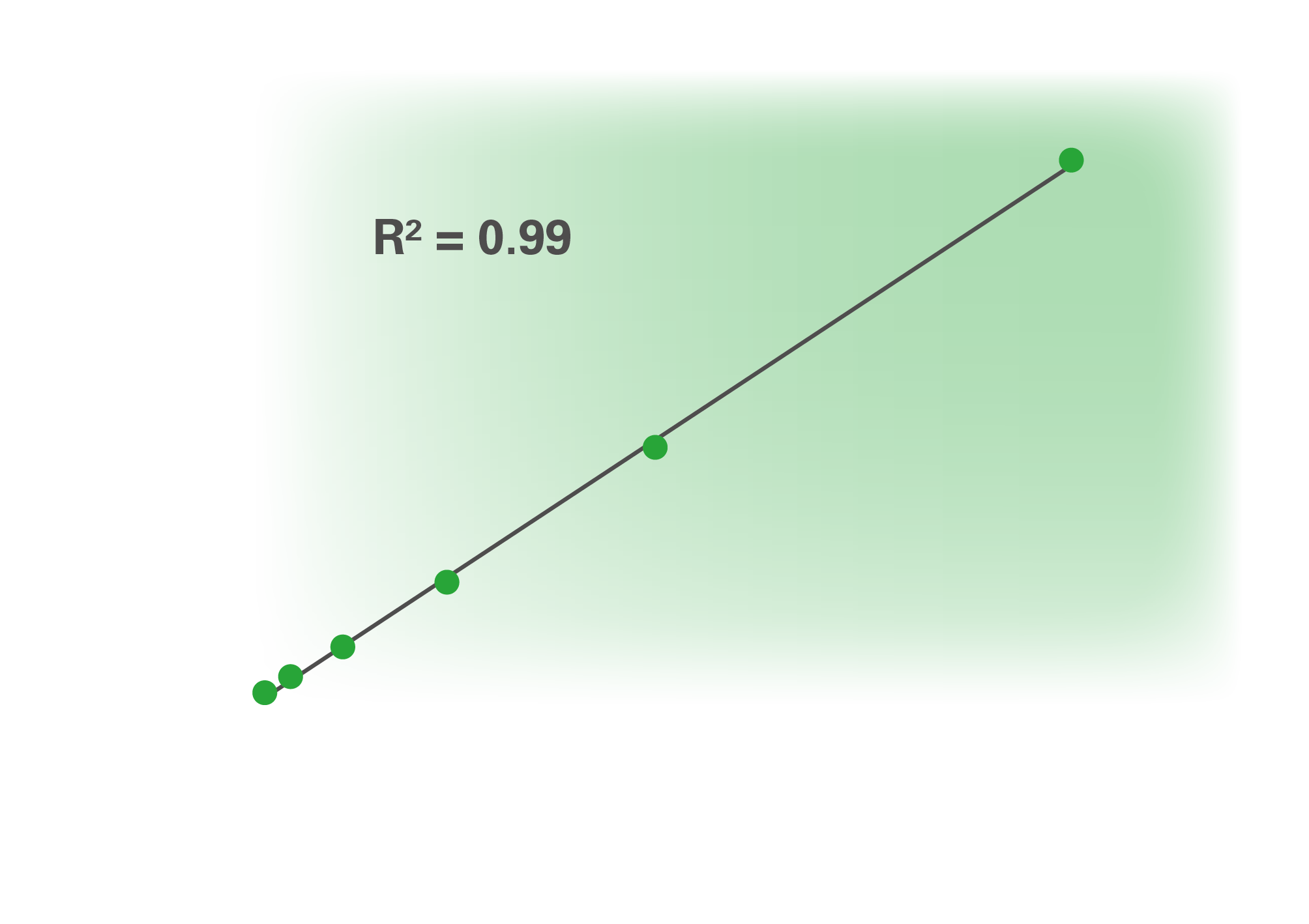
The World Health Organization (WHO) recently approved the first international reference material for standardization of results from anti-SARS-CoV-2 assays (National Institute for Biological Standards and Control code: 20/136). The preparation contains a clearly defined antibody concentration, enabling comparison and harmonization of results between different test systems. We rapidly aligned our quantitative S1-based ELISA to this standard. The CE-marked and fully automatable Anti-SARSCoV-2 QuantiVac ELISA (IgG) now provides quantification of the antibody concentration in standardized units (binding antibody units, BAU/ml) using a six-point calibration curve. The quantitative ELISA was tested with serial dilutions of the WHO reference serum and yielded an excellent correlation with the standard (coefficient of determination R2 = 0.99)
Detection of neutralizing antibodies
Antibodies against S1/RBD have a strong neutralizing effect, due to their ability to inhibit S1/RBD binding to ACE2 and thus prevent infection. Further specialized tests enable verification of the neutralizing function of the antibodies. The gold standard for determining neutralizing antibodies is the plaque reduction neutralization test (PRNT) or virus neutralization test (V-NT). These tests are, however, time consuming and expensive to perform. Moreover, they require biosafety level three (BSL-3) laboratory conditions owing to the use of pathogenic virus. A new CE-marked surrogate virus neutralization test (sVNT) enables fast and economical determination of the inhibiting effect of SARS-CoV-2 antibodies. The SARS-CoV-2 NeutraLISA is based on competitive binding between neutralizing antibodies in the patient samples and labelled ACE2 receptors to recombinant S1 coated onto the microplate wells. Results are evaluated qualitatively with a cut-off range of 20% to 35% inhibition. The ELISA is linear in the range up to 67% inhibition and shows an excellent correlation with the WHO international reference standard in this range (coefficient of determination R2 = 0.98). The cut-off area of the test corresponds to a value of around 62 IU/ml. The assay is much quicker to perform than the PRNT, requiring only two hours rather than days. Moreover, it does not require the use of BSL-3 conditions, making it suitable for routine diagnostics. It can be fully automated and is therefore ideal for high-throughput requirements.
High test specificity
The S1 antigen is among the least evolutionarily conserved of the coronavirus family. Therefore, antibodies against it are highly specific for SARS-CoV-2, resulting in a very high test specificity. The specificities of the Anti-SARS-CoV-2 QuantiVac ELISA (IgG) and the SARS-CoV-2 NeutraLISA were investigated using cohorts of control sera that were positive for antibodies against other human pathogenic coronaviruses or other infectious agents, as well as sera from healthy blood donors collected prior to the COVID-19 pandemic. The Anti-SARS-CoV-2 QuantiVac ELISA (IgG) demonstrated a specificity of 99.8% (n=1458) and the SARS-CoV-2 NeutraLISA a specificity of 99.7% (n=759).
Comparison with PRNT
Results from the Anti-SARS-CoV-2 QuantiVac ELISA (IgG) and the SARS-CoV-2 NeutraLISA were compared to those from PRNT using 74 sera from patients with a past confirmed SARS-CoV-2 infection (Table 1). The Anti-SARS-CoV-2 QuantiVac ELISA (IgG) exhibited a 97.3% agreement with the PRNT, confirming that the anti-S1/RBD antibodies detected with this ELISA were in most cases neutralizing antibodies. The SARS-CoV-2 NeutraLISA also gave a high agreement of 98.6% with the PRNT. Additionally, results from the Anti-SARSCoV-2 QuantiVac ELISA (IgG) and the SARS-CoV-2 NeutraLISA were compared with each other. The agreement between the tests amounted to 99.1% excluding borderline sera. In isolated cases results from the neutralization test and quantitative anti-S1 IgG determination may be discrepant. Possible reasons include the presence of IgA antibodies with an inhibiting function, which are detected in addition to the IgG by the neutralization test but not by the Anti-SARS-CoV-2 QuantiVac ELISA (IgG). In these cases it may be helpful to repeat the investigations 7 to 14 days later.
Multiplex detection of coronavirus antibodies
Antibodies against different SARS-CoV-2 antigens and against seasonal human coronaviruses (HCoV) can also be determined in parallel using immunoblot. The EUROLINE Anti-SARS-CoV-2 Profile (IgG) contains individual bands of SARS-CoV-2 spike protein domain S1 (including the RBD), domain S2 and nucleocapsid protein (NP) as well as bands of the NPs from four HCoV (HCoV-HKU1, HCoV-OC43, HCoV-NL63 and HCoV-229E). The profile thus enables differentiated detection of anti-SARS-CoV-2 IgG antibodies as well as antibodies against other HCoV in one test. The determination of antibodies against NP antigens from seasonal coronaviruses is intended for information purposes only
Determination of T-cell reactivity
T-cell immunity, particularly to the spike protein, appears to be associated with strong protection, even in patients who do not exhibit detectable levels of antibodies. According to current knowledge, about 10% of symptomatic and 40% of asymptomatic patients lose their IgG antibodies, so that the immune response may only be detectable via the T-cell response. Cellular immunity conferred by long-lasting T-cells can be measured using the SARS-CoV-2 interferon-gamma release assay (IGRA). The IGRA is performed on heparinized whole blood samples, circumventing the need to prepare purified peripheral mononuclear cells (PBMCs). The T-cells in the patient blood samples are stimulated based on specific viral spike protein in the provided tubes, and the interferongamma released by the T-cells is subsequently determined using a fully automated quantitative ELISA. The EUROIMMUN IGRA is currently available for research use only.
Perspectives
After more than a year of the COVID-19 pandemic, the roles of antibodies and T-cells in SARS-CoV-2 immunity are still not fully clarified. Further studies using tools such as the assays described here will help improve knowledge of SARS-CoV-2 immune responses, supporting development and optimization of vaccines, application of therapeutics and disease impact surveillance. There are currently 320 COVID-19 vaccines in development, with 97 in clinical trials and 17 in use worldwide [1]. The majority of these are based on the S1 protein. Comprehensive analysis of S1-specific humoral and cellular immune reactions following vaccination is important for fine-tuning immunization strategies with regard to dosage, boosters and individual circumstances such as previous infection. Cross protection between different SARS-CoV-2 variants following infection or vaccination and the durability of immune memory are also major topics of current research. Analysis of antibody and T-cell responses is additionally useful in epidemiological studies to elucidate the spread of COVID-19, especially given that many mild or asymptomatic infections remain undetected.
The expert
Jacqueline Gosink PhD
EUROIMMUN AG, 23560 Lubeck, Germany
References
1. COVID-19 vaccine tracker [website]. London School of Hygiene and Tropical Medicine (https://vac-lshtm.shinyapps.io/ncov_ vaccine_landscape/)