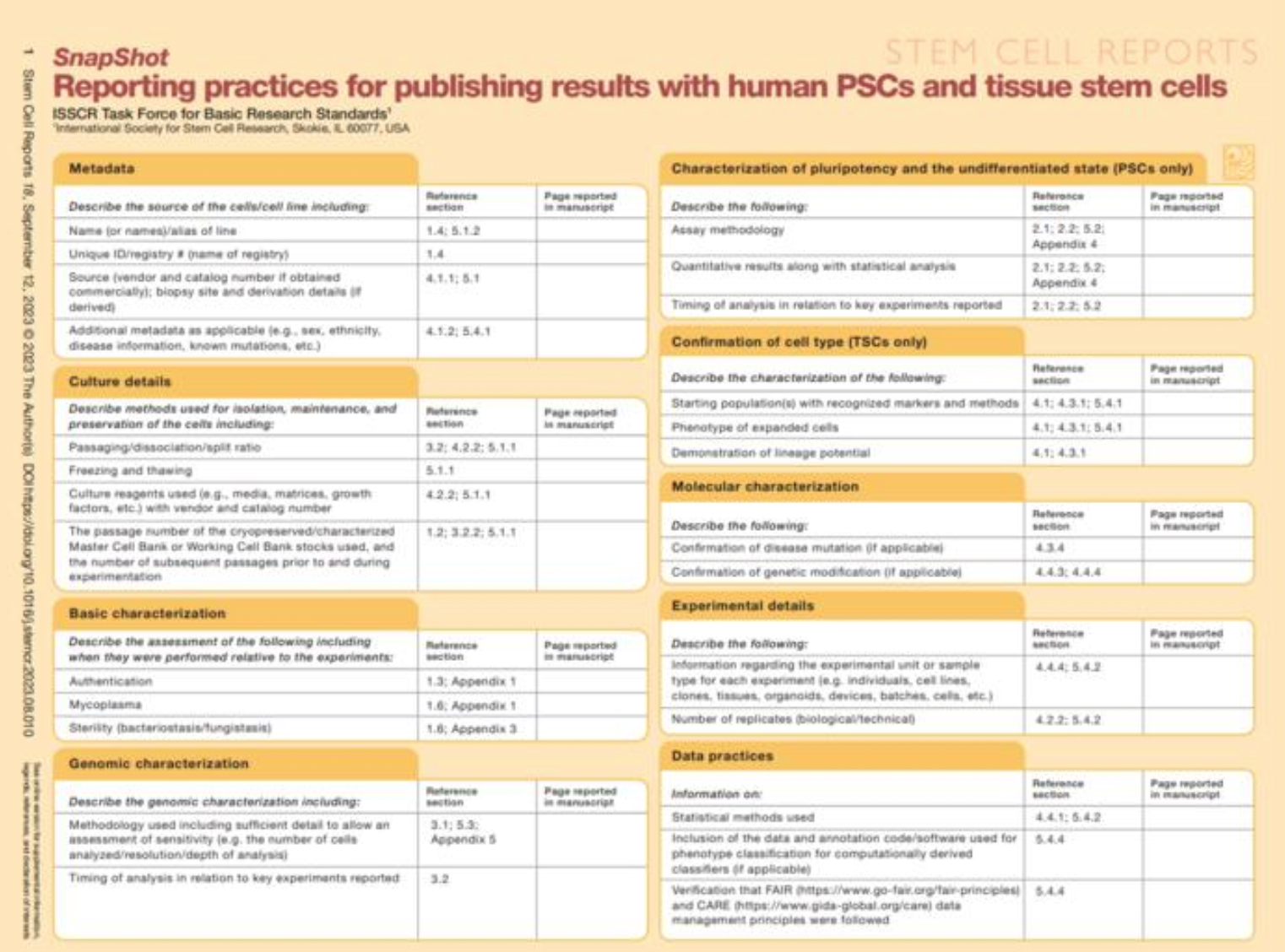
ISSCR introduces checklist to promote global best practices for human stem cell research
ISSCR introduces checklist to promote global best practices for human stem cell research Recommendations from the Standards for Human Stem Cell Use in Research [1], published in June by the International Society for Stem Cell Research (ISSCR), include a publishing “checklist” that is now being used by laboratory scientists and implemented in the review process by scientific publishers.
“The goal of this checklist is to increase clarity and transparency in the reporting of certain key quality control measures unique to the field of stem cell research,” said Martin Pera, Editor in Chief of Stem Cell Reports, who served on the international task force that developed the standards. “This is similar in format to editorial policy checklists already in use at many journals, enabling authors to disclose the critical experimental details of their research for review and potential replication.”
While some of the recommended practices are broadly applicable to the use of cultured cells, the ISSCR Standards and the accompanying checklist additionally address issues specific to the use of tissue and pluripotent human stem cells. The checklist has nine reporting categories and encourages shared language, consistency
in materials, and clear reporting practices aimed at addressing ongoing issues shared by the stem cell scientists.
The ISSCR Standards Initiative, launched in 2021, is led by an 11-member steering committee comprising international experts. The society pursued this initiative, recognizing the opportunity to establish best practices and reporting recommendations for pluripotent and adult stem cell research to improve rigor in the field. The basic and preclinical standards, released in June 2023, will be followed by clinical standards, likely in 2025.
References
1. https://www.isscr.org/standards-document