Laboratory biomarkers of rheumatoid arthritis
Rheumatoid arthritis is a systemic autoimmune disease that is an important socio-economic health problem. Recent evidence about the immunopathogenesis of this disorder might open new perspectives for a more appropriate laboratory approach. In this review, our attention is focused on the clinical relevance and appropriateness of laboratory biomarkers correlated with early diagnosis, prognosis, evolutive aspects of the disease and therapeutic efficacy.
by Prof. D. P. Foti, Dr E. Palella, F. Accattato, M. Greco, Prof. E. Gulletta
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory polyarthritis that can affect any synovial-lined diarthrodial joint, especially the wrist and the small joints of the hand. RA evolves as progressive articular damage leading to joint deformities and may be accompanied and complicated by several extra-articular manifestations [1]. The onset of RA clinical manifestations is often between the 4th and 5th decades of life, although a second peak of incidence is reported between 60 and 70 years of age. The prevalence is of 0.5–1% in industrialized countries, reaching the rate of 5% in women over 55 years. Following the 2010 RA classification criteria, the target population to be tested includes patients with at least one joint with a definite clinical synovitis that cannot be explained by another alternative disease. The classification criteria for RA is a score-based algorithm: a score of at least 6/10 obtained by the sum of scores for each category A–D (Joint involvement, Serology, Acute-phase reactants, Duration of symptoms) is needed to classify a patient as having definite RA [2].
RA is a multifactorial disease, in which environmental and genetic factors seem to play a role in the susceptibility and evolution of illness [3]. Several studies have shown a close genetic association with antigens of the MHC-II, in particular HLA-DRB1 [4], and PTPN22 that encodes the lymphoid tyrosine phosphatase (LYP), which is a critical negative regulator of signalling through the T cell receptor [5]. It is known that molecular targets of rheumatic autoimmune reaction are proteins that undergo post-translational modification typically associated with inflammation and apoptosis, such as citrullination and keratinization. Self-antigens (collagen, proteoglycans, rheumatoid factor and citrullinated proteins) probably play a role in the chronic evolution of the process, whereas super-antigens may be involved in the onset of illness. Pro-inflammatory substances released by cells from the immune system (GM-CSF, IL-1, IL-6, TNFα and its receptor, IL-17, IL-20, IL-21, IL-23) maintain the inflammatory process and contribute to the chronic damage [6].
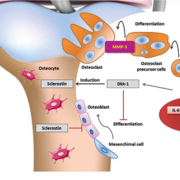
Most recent data on the pathogenetic mechanisms have led to a new laboratory approach on the choice of proper biomarkers [7] useful for each phase of clinical decision making (prediction, diagnosis, prognosis, monitoring therapeutic efficacy or adverse effects), in order to improve the patient management (fig. 1).
Biohumoral markers
Rheumatoid factor
Rheumatoid factor (RF) is an antibody directed against the Fc portion of immunoglobulin G (IgG). The evaluation of its isotypes has been used to enhance the serological diagnosis of RA, although in seropositive RA patients, the levels of RF are not related to either bone damage or status of the disease. However, it is not specifically associated with RA as it may be present in patients affected by other autoimmune or infectious diseases, and even in healthy elderly subjects. For several years, RF has been proposed as a useful tool for classifying patients as positive or negative at onset of disease and monitoring biological therapies [8].
Matrix metalloproteinases
Several proteolytic enzymes, including matrix metalloproteinases (MMP-1, -2, -3 and -9), cysteine proteinases (cathepsin B, H, L), serine proteinase (elastase, PA, cathepsin G) and aspartic acid proteinase (cathepsin D), play a role in the pathogenesis of RA. Among these, the metalloproteinases represent a family of important factors which cause the destruction of articular tissue. MMP-3 (stromelysin-1), expressed by synovial and articular cells, fibroblasts, chondroblasts and osteoclasts, can be a very useful marker for prediction of joint destruction. It acts upon the extracellular components of cartilage, such as fibronectin, collagen IV and V, elastin, proteoglycans, or even together with other MMPs in the disruption of cartilage. It is present in synovial fluid during the active phase of disease and its levels correlate with serum concentrations, independently from the patient’s age and severity of the disease. MMP-3 levels are strongly associated with disease activity, inflammatory markers and cartilage breakdown, indicating that it represents a potential biomarker of severity and progression to disabling disease [9].
A Japanese study has demonstrated that serum MMP-3 levels can be considered a predictor of joint destruction in RA, and its assessment could be useful in routinely evaluated outcome in the follow-up of RA patients [10].
Autoantibodies
Currently, several papers emphasize the importance of identifying a complete profile of RA-associated antibodies to improve the early diagnosis of disease and provide prognostic and theragnostic indications. The immunological profile consists of the definition of haplotype, evaluation of the cytoplasmic pattern of antinuclear autoantibodies (ANA), anti-citrullinated peptides antibodies (ACPA), and measurement of plasma levels of Th1 and Th2 cytokine networks. ACPA (vimentin, type II collagen, alpha-enolase and fibrinogen) are specific for RA and are associated with typically distinct clinical behaviour and genetic background. These antibodies can be present in serum years before the appearance of clinical symptoms and are highly specific and extremely useful for diagnosing RA. In this regard, ACPA serological positivity could be considered the most specific biomarker for RA, although these antibodies are not appropriate for monitoring disease progression [11].
RA and non-RA patients could be discriminated by a cyclic citrullinated peptides (CCP) antibody evaluation. Anti-CCP2 antibodies (IgG and IgA isotypes), a subset of ACPA, foresee the onset and development of RA, with the highest predictive value seen for IgG anti-CCP2 autoantibodies. This analytical data can have a higher positive predictive value in an at-risk rather than in a general population, thus the evaluation of the haplotype profile can improve the early diagnostic outcome.
Anti-CCP2 antibodies demonstrate the best diagnostic performances for profiling, thus they must be used as a first-line screening for the identification of subgroups of patients. The use of multiplex assays may facilitate a wider implementation of profiling [12].
Cytokines
Cytokines regulate many biological processes, including inflammatory and immune responses. An imbalance between pro- and anti-inflammatory cytokines or their uncontrolled production by activated immune cells can play a crucial role in regulating inflammatory diseases, such as RA.
Patients affected by RA have increased serum levels of several cytokines and chemokines years before the onset of symptoms of joint disease. Cytokine measurement by microarray is useful to evaluate the profile of pro- or anti-inflammatory molecules (IL-1β, IL-6, TNF-α, IL-10, VEGF, MCP-1, IL-17). In RA, Th-17 cells have been shown to play a central role by secreting IL-17, which activates a number of cell types involved in the pathogenesis of RA, including synovial fibroblasts, monocytes, macrophages, chondrocytes and osteoblasts [13]. The immune response during RA can also be modulated by Treg lymphocytes. These cells can be well characterized by cytofluorimetric assay by targeting specific markers (CD4+CD25high FoxP3+). The balance between Th-17 and Treg cells is a key point in autoimmune response. In general, Th-17 cells promote autoimmunity, whereas Treg cells protect against the occurrence of autoimmune diseases. Recent data have shown that IL-6 and TNF-α, by triggering Th17-cells, may alter the Th17/Treg balance, thereby promoting the autoimmune response. In this context, innovative therapies using anti-TNF-α and anti-IL-6 biological drugs, by decreasing the Th17/Treg ratio, have been shown to cause a clinical improvement in RA patients [14].
Multiparametric approaches in RA diagnosis and management
Pathogenic and clinical evidence suggest a new approach for laboratory medicine to evaluate patients in all different phases of RA progression. The American Rheumatism Association guidelines recommend that baseline laboratory evaluation include a complete blood cell count with differential, RF, erythrocyte sedimentation rate (ESR) and/or C-reactive protein (hsCRP), renal and hepatic function assessment. These laboratory findings may also be used to monitor the disease course in association with ANA and Anti-CCP antibodies [15]. In order to completely and correctly evaluate RA patients, several studies suggest combining the cytokine profile and MMP-3 measurements with conventional tests. The measurement of cytokines by multiparametric microarray is needed to completely evaluate the immunological response, the activation of Th1 or Th2 cells, the cytokine network and the stimulation of Th17 cells. MMP-3 can be considered an effective biomarker of disease aggressiveness and progression. Recently, by using a Venn diagram to predict potentially useful laboratory analytes, Curtis et al. have validated an algorithm with 12 biomarkers to obtain a multi-biomarker disease activity (MBDA) score for RA patients, with no effects from common comorbidities [16]. This complete laboratory profiling may allow a correct and personalized therapeutic treatment and a prognostic evaluation. In the future, the application of genomics and proteomics arrays will provide significant improvements in the characterization of the individual patient’s status at diagnosis and the response to therapeutic treatments.
References
1. Cavagna L, Boffini N, Cagnotto G, et al. Atherosclerosis and rheumatoid arthritis: more than a simple association. Mediators Inflamm. 2012; 2012: 147354.
2. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010; 62: 2569–2581.
3. Pincus T, Kavanaugh A, Sokka T. Benefit/risk of therapies for rheumatoid arthritis: underestimation of the “side effects” or risks of RA leads to underestimation of the benefit/risk of therapies. Clin Exp Rheumatol. 2004; 22(5 Suppl 35): S2–11.
4. Deane KD, El-Gabalawy H. Pathogenesis and prevention of rheumatoid disease: focus on preclinical RA and SLE. Nat Rev Rheumatol. 2014; 10(4): 212–228.
5. Fiorillo E, Orrú V, Stanford SM, et al. Autoimmune-associated PTPN22 R620W variation reduces phosphorylation of lymphoid phosphatase on an inhibitory tyrosine residue. J Biol Chem. 2010; 20: 26506–26518.
6. Burmester GR, Feist E, Dörner T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat Rev Rheumatol. 2014; 10: 77–88.
7. Smolen JS, Alehata D, Redlich K. The pathogenesis of rheumatoid arthritis: new insights from old clinical data? Nat Rev Rheumatol. 2012; 8(4): 235–243.
8. Can M, Najip A, Yılmaz N, et al. Immunoglobulin subtypes predict therapy response to the biologics in patients with rheumatoid arthritis. Rheumatol Int. 2013; 33(6): 1455–1460.
9. Mamehara A, Sugimoto T, Sugiyama D, et al. Serum MMP-3 as predictor of joint desctruction in RA, treated with non-biological diseases modifying anti-rheumatic drugs. Kobe J Med Sci. 2010; 56(3): E98–107.
10. Shinozaki M, Inoue E, Nakajima A, et al. Elevation of serum matrix metalloproteinase-3 as a predictive marker for the long-term disability of rheumatoid arthritis patients in a prospective observational cohort IORRA. Mod Rheumatol. 2007; 17(5): 403–408.
11. Jaskowski TD, Hill HR, Russo KL, et al. Relationship between rheumatoid factor isotypes and IgG anti-cyclic citrullinated peptide antibodies. J Rheumatol 2010; 37(8):1582–1588.
12. Conrad K, Roggenbuck D, Reinhold D, Dörner T. Profiling of rheumatoid arthritis associated autoantibodies. Autoimm Rev 2010; 9(6): 431–435.
13. Samson M, Audia S, Janikashvili N, et al. Brief report: inhibition of IL-6 function corrects Th17/Treg imbalance in rheumatoid arthritis patients. Arthritis Rheum. 2012; 64(8): 2499–2503.
14. Miossec P. Interleukin-17 in rheumatoid arthritis: if T cells were to contribute to inflammation and destruction through synergy. Arthritis Rheum. 2003; 48: 594–601.
15. Singh A. 2012 Update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res. (Hoboken) 2012; 64: 625–639.
16. Curtis JR, van der Helm-van Mil AH, Knevel R, et al. Validation of a novel multibiomarker test to assess rheumatoid arthritis disease activity. Arthritis Care Res. (Hoboken) 2012; 64(12): 1794–1803.
17. Imboden JB. The immunopathogenesis of rheumatoid arthritis. Annu Rev Pathol. 2009; 4: 417–434.
The authors
Daniela P. Foti MD, PhD; Eleonora Palella MD; Francesca Accattato Bs Sci; Marta Greco Bs Sci; Elio Gulletta* MD
Dept. of Health Sciences, University Magna Grecia, Catanzaro, Italy
*Corresponding author
E-mail: gulletta@unicz.it