Latest studies confirm TnI assay performance in early rule-out protocols for AMI
The burden of cardiovascular disease continues to take its toll in terms of diminished quality of life, reduced life expectancy, and the direct and indirect medical costs of treating and caring for patients. This is in spite of downward trends in mortality in the US and Western Europe, especially in the two decades leading up to the year 2000.
by Bernard Cook, PhD
This decline could, in part, be attributed to the success of patient educational campaigns to reduce smoking, lower levels of cholesterol and blood pressure, and encourage lifestyle changes in exercise and diet. Alongside the wider use of effective medications such as cholesterol-lowering statins, these steps are said to have contributed to an observed decline in coronary heart disease (CHD) deaths [1].
However, the rate of improvement is being affected by other, conflicting trends, such as increasing extreme obesity, sedentary lifestyles, the prevalence of hypertension and the rise in type 2 diabetes mellitus even in children.
The World Health Organization first defined myocardial infarction (MI) in 1979 based on clinical presentation, ECG results and elevated blood enzymes such as total CK and the CK-MB isoform. However, there were no standardized definitions, with clinicians defining MI differently, even within the same hospital. The arrival of cardiac troponin assays in the 1990s opened the door for a much needed reappraisal. It was discovered that a greater sensitivity and specificity for MI diagnosis could be achieved by using troponin (a more cardiac-specific marker than CK-MB) and lower cut-offs, presenting the possibility of using the rise and fall of troponin levels to establish ‘early rule-out protocols’.
Highly sensitive troponin is biomarker of choice
Performance expectations have risen as the sensitivity of troponin assays has improved. Nowadays, the modern, highly sensitive troponin assay has become the gold standard biomarker for the early diagnosis of an acute MI, with standards benchmarked by recent international guidelines.
The first steps towards international conformity were taken in 2000 when the European Society of Cardiology (ESC) and the American College of Cardiology (ACC) collaborated on a redefinition of MI. For the first time this gave a central role to the use of biomarkers such as troponin [2]. By 2007, this collaboration had expanded to include the American Heart Association (AHA) and the World Heart Federation (WHF) and saw the publication of the first universal definition of MI. This further established troponin as the preferred biomarker when diagnosing a heart attack [3].
Five years later, in 2012, the Third Universal Definition of Myocardial Infarction [4] laid down the criteria for the contemporary use of troponin assays by today’s clinicians to reduce the time it takes to rule out acute myocardial infarction (AMI). Crucially, this defined an increased cTn concentration as a value exceeding the 99th percentile of a normal reference population apparently free from heart disease [5, 6].
Establishing upper reference limits
The current definition states that the 99th percentile limit should be determined using a healthy population [5, 7]. It is acceptable to confirm this cut-off from information published in peer-reviewed literature or in the assay manufacturer’s own product literature. Further, troponin assays should demonstrate optimal precision with a coefficient of variation of 10% or less at the 99th percentile value and high sensitivity assays should be able to detect troponin in at least 50% of the reference population [7, 8, 9]. Troponin assays with an imprecision greater than 20% CV at the 99th percentile do not fit the criteria for contemporary use.
In today’s current clinical practice, when patients present to an emergency department with chest pain and acute coronary syndrome is suspected, the requirement for the highly sensitive troponin (Tn) assays, such as Beckman Coulter’s AccuTnI+3 assay, is to rule out non-ST-segment-elevation myocardial infarction (NSTEMI) as quickly as possible. Further, distinguishing acute from chronic c-Tn elevations requires serial measurements to detect significant changes [5].
There are conflicting positions about how to best establish a 99th percentile upper reference limit (URL) for troponin. The first maintains that the URL study should include younger subjects, and should not include subjects with potential cardiovascular disease or cardiac risk factors. The second contends the study should include subjects from a population that represents the intended use for troponin: patients whose demographics reflect those of subjects presenting to the emergency department, including older individuals without known cardiovascular disease. Additionally, other methods for selecting a cardiac-healthy population have been employed, such as samples collected from apparently healthy blood donors.
In a study by Moretti et al to establish that the AccuTnI +3 assay demonstrates a coefficient of variation of 10%, the authors found that 62% of the apparently 330 healthy group of blood donors used in the study had measurable values of troponin between the Limit of Detection (LoD) and 99th percentile values [10]. There were no significant differences related to gender and no correlation between cTnI and age.
Predictive accuracy at early observation times
Storrow et al conducted a multicentre, prospective study, involving more than 1,900 patients, to investigate and compare clinical performance at pre-defined serial sampling intervals: on admission/at 1-3 hours/3-6 hours and 6-9 hours. Patients selected from 14 centres were those presenting with chest pain or equivalent ischemic symptoms suggestive of acute coronary syndromes [11]. Results from this study reinforced current clinical practice that troponin testing provides a high degree of accuracy at early observation times, on admission and three hours later, only needing to be repeated after six hours when clinical suspicion remains high.
The findings, published late 2014 in Clinical Biochemistry, compared emergency department TnI serial sampling intervals to determine optimal diagnostic thresholds, and reported on representative diagnostic performance characteristics for early rule-in and rule-out of MI [11]. Diagnosis was adjudicated by an independent central committee of cardiologists. Study samples were tested using Beckman Coulter’s AccuTnI+3 assay at four independent testing facilities.
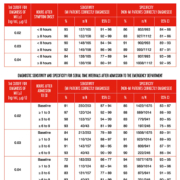
Specific results from Storrow showed that TnI ≥0.03 ng/mL provided 96.0% sensitivity and 89.4% specificity at 1-3 hours after admission, and 94.9% sensitivity and 86.7% specificity at 3–6 hours. When troponin levels were <0.03 ng/mL, being able to give a negative predictive value (NPV) depended on knowing the time symptoms started. If it was determined that symptoms started approximately eight hours before admission or examination, the NPV was 99.1%. Testing at 1-3 hours gave a NPV of 99.5%; and 99.0% at 3-6 hours when TnI is >0.03 ng/mL. However, Storrow noted that 50–58% of patients with troponin levels of ≥0.03 ng/mL were diagnosed with MI, depending on the time symptoms started or admission.
Positive predictive values emphasize the importance of taking serial samples and observing rising or falling patterns of the delta TnI when low cut-offs are used. Storrow noted that even a single elevated TnI value increased the risk of MI. As TnI values rose, the probability of MI increased, with values ≥0.20 ng/mL associated with an almost 90% probability [11].
Importance of establishing absolute delta values
A change in serial troponin values (delta) can be reported as a percentage or absolute concentrations between the repeat measurements. However, serial measurements must be calculated with values from the same cTn assay [12]. The larger the value set for the delta, the higher the specificity (and the lower the sensitivity) for acute cardiac injury including AMI [13], and the smaller the value set, the higher the sensitivity.
In a separate report using the AccuTnI+3 assay, Storrow confirmed that absolute delta performed significantly better than relative delta at each time interval; for example, at 1-3 hours (AUC were 0.84 vs 0.69), 3-6 hours (0.85 vs 0.73), and 6-9 hours (0.91 vs 0.79) [14]. Current recommendations propose ≥20% delta within 3-6 hours; however, in this study, results were optimized using an absolute delta of 0.01 or 0.02 ng/mL.
Being able to determine the degree of serial change in high sensitive troponin assay concentrations seems to be the most accurate way of differentiating between those patients suffering AMI and those with more chronic heart conditions. Currently, there is no official consensus on a way of establishing or confirming delta values. Until this is in place, the US Task Force in Clinical Applications of Cardiac Bio-markers recommends that institutions agree on a delta value based on available peer-reviewed data for individual assays and then modify based on empirical findings and feedback [15].
Another study of 874 patients, published in the International Journal of Cardiology, used the Beckman Coulter AccuTnI assay to demonstrate that an algorithm incorporating cTnI concentration and delta cTn values with this assay could allow accurate diagnosis of AMI within two hours from presentation and an earlier rule-out of AMI in the majority of patients.
Cullen et al assessed the accuracy of delta cTn at two and six hours compared to the cTn concentration above the 99th percentile reference value for AMI in a prospective study of adult patients presenting with symptoms suggestive of possible acute coronary syndrome [16]. The area under the ROC curve for diagnosing AMI at two hours was 0.89 [95%CI, 0.84–0.95] for absolute delta cTn versus 0.79 [95%CI 0.73–0.85] for the relative change. Specificity and PPV at two hours were optimized using a delta cTnI ≥0.03 μg/L (95.8% [95%CI 94.1–97.0] and 61.4% [95%CI 50.9–70.9] respectively). Sensitivity and NPV for AMI were optimized using the 99th percentile with the addition of a delta of 0.03 μg/L (97.1% [95%CI 90.2–99.2] and 99.7% [95%CI 99–99.9] respectively).
Labs to adopt lower high sensitive assay cut-offs
With the newer highly sensitive troponin assays, the resulting shift to lower cTn cutoffs will increase the number of patients that are monitored for MI, and will also identify patients with elevated cTn due to other conditions. Published guidance now recommends that all manufacturers demonstrate the true clinical performance of their cTn assays in the contemporary clinical setting through an appropriately designed clinical study [17]. Out-of-date clinical cut-offs and diagnostic criteria may not accurately diagnose MI in some instances. It may take time for laboratories to adopt lower cut-offs, but doing so will ultimately improve patient care [18].
References
1. Ford ES, Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002: concealed leveling of mortality rates. J Am Coll Cardiol. 2007 Nov 27; 50(22):2128-32. Epub 2007 Nov 13.
2. Myocardial Infarction Redefined – a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur. Heart J 2000; 21: 1502-1513. J Am Coll Cardiol 2000; 36: 959-969.
3. Thygesen K, Alpert JS, White HD; Joint ESC/ACC/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Eur Heart J 2007; 28: 2525-38.
4. Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. European Heart Journal 2012; 33: 2551-2567. Available online at: http://www.escardio.org/guidelines.
5. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J 2012; 33: 2251–2267.
6. Morrow DA, Cannon CP, Jesse RL, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Clin Chem 2007; 53: 552–574.
7. Collinson PO, Heung YM, Gaze D, Boa F, Senior R, Christenson R, et al. Influence of population selection on the 99th percentile reference value for cardiac troponin assays. Clin Chem 2012; 58:219-25.
8. Apple FS, Collinson PO, and for the IFCC Task Force on Clinical Applications of Cardiac Biomarkers: analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 2012; 58:54-61.
9. Apple FS. Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common, presumably healthy, population. Clin Chem 2012; 58:1574-81.
10. Moretti M et al. Analytical performance and clinical decision limit of a new release for cardiac troponin I assay. Ann Clin Biochem, April 7, 2014.
11. Storrow AB, et al. Diagnostic performance of cardiac Troponin I for early rule-in and rule-out of acute myocardial infarction: Results of a prospective multicenter trial. Clinical Biochemistry 2014; e-pub ahead of print.
12. Keller T, Zeller T, Ojeda F, Tzikas S, Lillpopp L, Sinning C, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA 2011; 306:2684-93.
13. Korley FK, Jaffe ASJ. Preparing the United States for high-sensitivity cardiac troponin assays. J Am Coll Cardiol 2013; 61:1753-8.
14. Storrow AB, et al. Absolute and relative changes (delta) in troponin I for early diagnosis of myocardial infarction: Results of a prospective multicentre trial. Clin Biochem (2014), http://dx.doi.org/10.1016/j.clinbiochem.2014.09.012.
15. Task Force On Clinical Applications of Cardiac Bio-Markers. Using High Sensitivity Cardiac Troponin Assays in Practice. The International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). 2014. http://www.ifcc.org/media/259738/201405_TF_CB_IFCC_practice%20Summary.pdf.
16. Cullen L, Parsonage WA, Greenslade J, Lamanna A, Hammett CJ, Than M, et al. Delta troponin for the early diagnosis of AMI in emergency patients with chest pain. Int J Cardiol 2013;168:2602–8.
17. Letter to Manufacturers of Troponin Assays Listed with the FDA. Available online at: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm230118.htm.
18. Mills NL, Lee KK, McAllister DA, Churchhouse AMD, MacLeod M, Stoddart M, Walker S, Denvir MA, Fox KAA, Newby DE. Implications of lowering threshold of plasma troponin concentration in diagnosis of myocardial infarction: cohort study. BMJ 2012; 344: e1533.
The author
Dr Bernard Cook is Senior Scientific Manager, Beckman Coulter Diagnostics. He has co-authored several scientific papers and is actively involved in the diagnostics industry, which includes being the former chairman of the industry division of the American Association for Clinical Chemistry.