Lifespin launches commercial access to its metabolic profiler software and database for pharma and biobank services
Lifespin, based in Regensburg, Germany, with offices in Boston, Massachusetts, has launched a new commercial service that, for the first time, will provide the pharmaceutical and biobanking industry with
access to its proprietary metabolic database, as well as its advanced interpretive software to assist in various phases of drug research, development, and manufacturing.
Immediate applications of Lifespin’s commercial services will include quality control for synthetic/natural compounds, quantitative profiling of metabolites in liquid samples, monitoring of drug responses and organ-specific metabolic phenotyping, and precision nutrition to therapeutic drug monitoring and longitudinal treatment monitoring.
“Making our technology platform accessible to the pharma and biobanking industry will provide the field with deeper clinical insights and improve stratification of patient data in clinical trials,” said Dr Ali Tinazli, CEO of Lifespin. “The comprehensive and quantitative metabolic profiling services will enable pharmaceutical companies and others in the field to perform a range of precision phenotyping of cell or animal models and patient cohorts.”
Lifespin has built one of the largest and most comprehensive databases of metabolic health profiles across healthy and diseased individuals covering multiple age and gender groups as well as specific diseases in neurology, oncology, and inflammation. Leveraging this resource can offer significant benefits to researchers in the pharma and biobank fields.
“The human and financial costs of failed drug candidates or drugs being recalled after launch can be extraordinary,” Dr Tinazli said. “Our goal is to provide
the pharma and biobank scientists with yet another powerful resource in their development toolbox, including our advanced algorithms and database, to enhance their earlier phase analysis and to help better identify the strengths and weaknesses of compounds in study during research through clinical trials.”
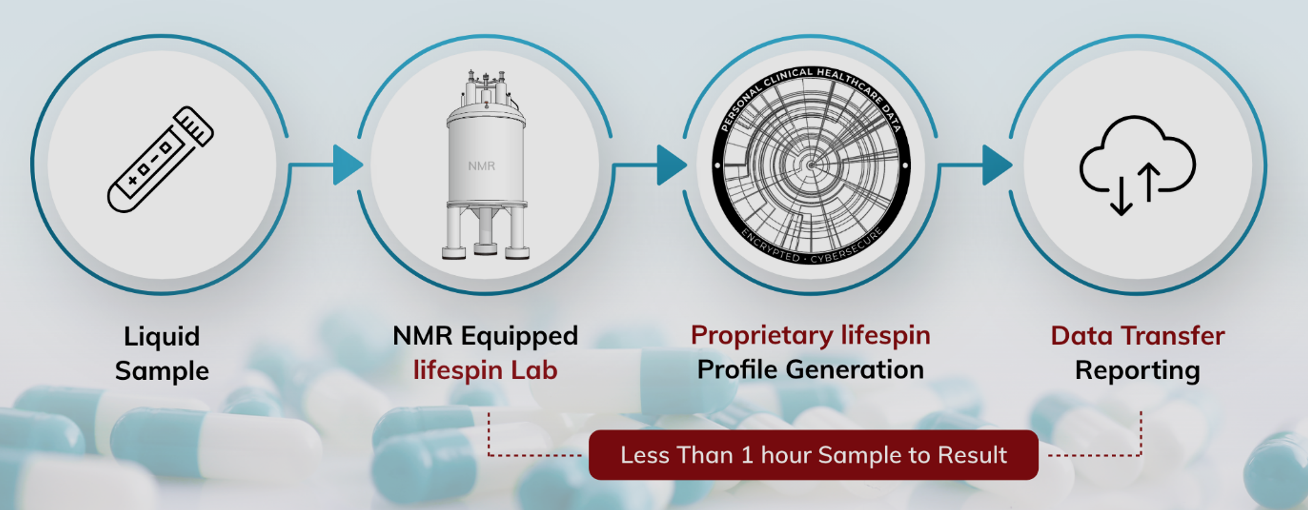
“Our proprietary platform enables quantitative and highly reproducible metabolic profiling with minimum time and low sample volume without the need for sample preparation,” said Dr Michael Helou, Head of Product and Business Development at Lifespin. “The highly precise, accurate and cost-effective analysis will be a powerful add-on for quality control, drug discovery as well as monitoring at any stage of drug development.”