Lipocalin 2 and brain-derived neurotrophic growth factor: biomarkers that link colorectal cancer and obesity?
Colorectal cancer is one of the most prevalent types of cancer and is the fourth most common cause of cancer mortality. Identification of non-invasive biomarkers representative of disease heterogeneity is critical for diagnosis of early stage disease when the chance for cure is greatest. This article discusses two such biomarkers, brain-derived neurotrophic growth factor and lipocalin 2, which also reflect key independent risk factors for the disease obesity.
by Dr K. Y. C. Fung, Dr B. Tabor, Prof. P. Gibbs, Dr J. Tie, Dr P. McMurrick, Mr J. Moore, Prof. A. Ruszkiewicz, Prof. A. Burgess, Dr L. Cosgrove
Biomarkers for colorectal cancer: current status
Colorectal cancer (CRC) is one of the most commonly diagnosed cancers worldwide where epidemiological studies have drawn strong correlations between its incidence and lifestyle factors [1, 2]. The incidence of CRC varies considerably with geographic region, where it is highest in affluent countries (e.g. in the USA, UK, Europe, Australia and New Zealand the incidence is approximately 20–45 per 100 000) and lowest in African and Asian countries (incidence of approximately 5–20 per 100 000) [2]. In countries with increasing industrialization such as Japan, Korea and Singapore, the incidence of CRC is rapidly approaching that of high risk countries with a longer history of affluence [2]. For most sporadic CRC, the transformation from normal colonic mucosa to carcinoma is believed to occur over 10–15 yrs [3]. This relatively long time frame for disease development enables implementation of population screening programmes for disease detection as early stage diagnosis and removal of premalignant (adenoma or polyp) or early stage malignant disease (stage I) can either prevent the occurrence of CRC or significantly increase the chance of a complete cure.
Ideally, diagnostic tests are robust and cost effective and biomarkers should have high sensitivity and specificity for the disease they are proposed to detect. Currently, colonoscopy is regarded as the ‘gold standard’ for CRC diagnosis (sensitivity and specificity greater than 95%) but it is expensive and invasive. Accordingly, low cost alternatives such as the fecal occult blood test (FOBT) and the fecal immune test (FIT) are currently in use in population screening programmes in a number of countries [4]. These tests detect the presence of blood in stool samples and have low specificity for CRC. Their low sensitivity also leads to high rates of false positive results and they do not reliably detect early stage disease [5, 6]. As a result, identification of suitable biomarker(s) with high sensitivity and specificity for CRC that can be included in a non-invasive test suitable for population screening is urgently required. Despite extensive research efforts, no single biomarker has been identified and it is becoming apparent that a panel of biomarkers panel reflecting the heterogeneity of the disease will be more effective.
Sporadic CRC is linked to multiple environmental risk factors, with obesity consistently demonstrated to be a significant and independent risk factor [1]. Brain-derived neurotrophic growth factor (BDNF) and lipocalin 2 (LCN2) are two protein biomarkers that have been implicated in both obesity and CRC. BDNF has been shown to have a key role in neural regulation of appetite and food intake control [7], where low BDNF levels in the hypothalamic region of the brain have been associated with decreased satiety and weight gain. There is also evidence indicating that serum BDNF levels are lower in patients with type 2 diabetes in comparison to controls [8]. Similarly, elevated levels of circulating LCN2 have been documented in obese men and women and in patients with metabolic syndrome [9]. With the aim of identifying a panel of biomarkers to identify individuals potentially at risk of developing CRC, we investigated the utility of BDNF and LCN2 as individual biomarkers and as a biomarker panel to determine if this combination provided higher sensitivity for CRC diagnosis.
BDNF and LCN2 as CRC biomarkers
We have previously reported on the utility of circulating BDNF and lipocalin as biomarkers for CRC [10, 11]. In these studies, enzyme-linked immunosorbent assays (ELISAs) were used to measure the concentrations of each biomarker in the sera of a cohort of CRC patients (n=97) and age/gender matched controls (n=99). In this cohort, the median BDNF concentration was found to be significantly lower (P<0.0001) in the control population (18.8 ng/mL, range 4.0–56.5 ng/mL) when compared to the CRC group (23.4 ng/mL, range 3.0–43.1 ng/mL). Conversely, in the same cohort, the median concentration of LCN2 was significantly higher (P<0.0001) in the CRC group (121.5 ng/mL, range 31.65–432.6 ng/mL) when compared to the control group (86.36 ng/mL, range 17.11–189.9 ng/mL). At 95% specificity, the sensitivity of BDNF was 18% [area under curve (AUC) 0.69, P<0.0001)] and the sensitivity of LCN2 was 31% (AUC 0.71, P<0.0001). Although both biomarkers performed equally well at separating CRC patients from the normal cohort (demonstrated by the AUC), neither biomarker when considered alone reached the desired sensitivity for clinical use as a diagnostic approach for CRC.
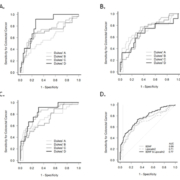
Figure 1 shows the receiver operating characteristic (ROC) curve for BDNF, LCN2 and for BDNF and LCN2 in combination. Table 1 summarizes the sensitivity at 95% specificity for BDNF and LCN2 individually and as a biomarker combination for each disease stage. LCN2 had consistently higher sensitivity than BDNF for diagnosing CRC overall and at each Dukes’ stage, and the LCN2 and BDNF combination does not appear to improve diagnostic efficacy. For example, at 95% specificity, the sensitivity was 33% for the LCN2 and BDNF combination (compared with 32% for LCN2).
Strategies for biomarker identification
Current strategies for CRC biomarker identification include identification of tumour specific biomarkers and biomarkers indicative of the disease process, such as inflammation, the immune response, angiogenesis, and metastasis. Investigators have also reported on the utility of biomarker combinations that include established tumour markers such as CEA and CA19-9 [12, 13]. These strategies have yielded many promising individual candidate markers and marker panels that have been tested in small cohort studies, but none has resulted in the sensitivity and specificity required for population based screening. This lack of success has been attributed to factors such as small sample size, over-representation of late stage disease in test cohorts leading to overestimation of biomarker sensitivity, and disease heterogeneity where CRC subsets with different genetic backgrounds have been characterized [14].
As part of our strategy, we have also considered biomarkers indicative of established risk factors such as obesity and type 2 diabetes. Inclusion of these biomarkers, or biomarkers that are indicative of other risk factors, should enable us to identify those individuals who may be at greater risk of developing the disease and hence improve our ability for earlier diagnosis. This is critical for reducing mortality and morbidity associated with CRC where the 5-year survival rate for patients with stage I disease is >90% in comparison to 5% at stage D. Currently, more than 50% of malignancies are detected at an advanced stage despite the implementation of screening programmes. Although the BDNF and LCN2 combination does not provide adequate sensitivity and specificity for use in a clinical setting, it is possible that a combination of (one of) these markers with a CRC tumour specific marker may yield the desired analytical performance.
Future directions
The lack of FDA approval for any biomarkers as a diagnostic for CRC highlights the challenges associated with discovery, verification and validation of biomarkers. While –omics technologies (e.g. genomics, transcriptomics and proteomics) have been, and continues to be, the primary tool for discovery of novel biomarkers, these efforts have largely focused on identification of tumour specific markers. Incorporation of biomarkers representative of other disease factors will likely improve our chances of identifying a panel of markers to successfully diagnose CRC. Furthermore, stratification of risk based on genotype or environmental/lifestyle factors together with a panel of molecular biomarkers may prove to be more successful than any one of these factors alone for early diagnosis.
Acknowledgements
We thank the Victorian Cancer Biobank (Melbourne, Victoria) for their assistance with sample collection and Ms Ilka Priebe for technical assistance with the ELISAs. This work was funded by the CSIRO Preventative Health National Research Flagship and the National Health and Medical Research Council (grant number 1017078).
References
1. World Cancer Research Fund / American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, DC: AICR 2007.
2. Jemal A, Bray F, et al. Global cancer statistics. CA Cancer J Clin. 2011; 61(2): 69–90.
3. Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990; 61(5): 759–767.
4. Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008; 103(6): 1541–1549.
5. Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Shiratori Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology 2005; 129(2): 422–428.
6. Parra-Blanco A, Gimeno-García AZ, Quintero E, Nicolás D, et al. Diagnostic accuracy of immunochemical versus guaiac faecal occult blood tests for colorectal cancer screening. J Gastroenterol. 2010; 45(7): 703–712.
7. Vanevski F, Xu B. Molecular and neural bases underlying roles of BDNF in the control of body weight. Front Neurosci. 2013; 7: 37.
8. Fujinami A, Ohta K, Obayashi H, Fukui M, et al. Serum brain-derived neurotrophic factor in patients with type 2 diabetes mellitus: Relationship to glucose metabolism and biomarkers of insulin resistance. Clin Biochem. 2008; 41(10–11): 812–817.
9. Wang Y, Lam KS, Kraegen EW, Sweeney G, et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem. 2007; 53(1): 34–41.
10. Brierley GV, Priebe IK, Purins L, Fung KY, et al. Serum concentrations of brain-derived neurotrophic factor (BDNF) are decreased in colorectal cancer patients. Cancer Biomark. 2013; 13(2): 67–73.
11. Fung KY, Priebe I, Purins L, Tabor B, et al. Performance of serum lipocalin 2 as a diagnostic marker for colorectal cancer. Cancer Biomark. 2013; 13(2): 75–79.
12. Herszényi L, Farinati F, Cardin R, István G, et al. Tumor marker utility and prognostic relevance of cathepsin B, cathepsin L, urokinase-type plasminogen activator, plasminogen activator inhibitor type-1, CEA and CA 19-9 in colorectal cancer. BMC Cancer 2008; 8: 194.
13. Shimwell NJ, Wei W, Wilson S, Wakelam MJ, et al. Assessment of novel combinations of biomarkers for the detection of colorectal cancer. Cancer Biomark. 2010; 7(3): 123–132.
14. Tao S, Hundt S, Haug U, Brenner H. Sensitivity estimates of blood-based tests for colorectal cancer detection: impact of overrepresentation of advanced stage disease. Am J Gastroenterol. 2011; 106(2): 242–253.
The authors
Kim Y. C. Fung1* PhD; Bruce Tabor1 PhD; Peter Gibbs2 MBBS, MD, FRACP; Jeanne Tie2 MD; Paul McMurrick3 MBBS, FRACS; James Moore4 MBBS, MD, FRACS; Andrew Ruszkiewicz5 MD, FRCPA; Antony Burgess6 PhD; and Leah Cosgrove1 PhD
1CSIRO, Preventative Health National Research Flagship, Australia
2Royal Melbourne Hospital, Melbourne, Australia
3Cabrini Hospital, Melbourne, Australia
4Royal Adelaide Hospital, Adelaide, Australia
5SA Pathology, Adelaide, Australia
6Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia
*Corresponding author
E-mail: Kim.fung@csiro.au