Looking at ANA testing from a different perspective
The HEp-2 immunofluorescence assay (IFA) for ANA screening is excellent for ruling out many connective tissue diseases, but a positive result seldom translates into a clinically meaningful diagnosis. A new automated, efficient, enzyme immunoassay for ANA screening provides reliable, objective information that can be applied clinically with confidence.
by James D. Peele, PhD
Introduction
For laboratory directors, the immunofluorescence assay (IFA) using HEp-2 cells is one of the most commonly ordered tests for antinuclear antibody (ANA) screening. ANA screening has been considered, along with patient history and physical examination, as one of the diagnostic tools for systemic autoimmune rheumatic diseases (SARD) for decades [1]. While the ANA screen is excellent for ruling out autoimmune diseases, possessing good sensitivity and negative predictive value (NPV), its specificity and positive predictive value (PPV) are less impressive. In the majority of cases, a positive ANA by IFA seldom translates into a clinically meaningful diagnosis. For example, 4 out of 5 positive HEp-2 results have no clinical relevance according to Peene and colleagues, who tested a large, consecutive cohort of serum samples [2]. More recently, 90% of patients referred to a tertiary rheumatology clinic for a positive ANA had no evidence of ANA-associated rheumatic disease [3]. Indeed, 31.7% of the normal population is ANA positive at a 1:40 serum dilution, 13% at 1:80, and 5% at 1:160 [4]. The financial impact for such unnecessary follow-up testing or specialist referrals continues to negatively impact any healthcare system.
These truths pose a dilemma for laboratory directors, who experience HEp-2 ANA testing as a laborious manual operation, which is designated as highly complex and requires skilled technologists [5]. In addition, consistency in performance of IFA testing and interpretation of results within and between labs is problematic, despite attempts at standardization. Interpreting titres and patterns can at times seem more art than science.
Using new ELISA-based technology (sometimes called EIA for enzyme immunoassay) to screen for multiple extractable nuclear antigen (ENA) markers, centromere and double-stranded DNA (dsDNA) has automated testing at Baptist Medical Center and greatly improved the PPV for our ANA screen. The most recent international recommendations for the diagnosis of SARD advise that a specific panel of laboratory tests be ran to include ANA, anti-dsDNA and anti-ENA antibodies, [6] which collectively make up what we commonly call our ANA screen. Our experience illustrates how evolving technology can work hand-in-hand with clinical expertise to improve the diagnostic process.
Comparing EIA with conventional ANA testing
The long tenure of HEp-2 ANA testing means that rheumatologists have grown accustomed to the test’s utility and to its limitations. The American College of Rheumatology (ACR) emphasizes that “A positive ANA test means autoantibodies are present. By itself, a positive ANA test does not indicate the presence of an autoimmune disease or the need for therapy” [7]. This understanding may not be applied among primary care physicians, who often order the test before referral. Some specialists actively discourage the use of IFA as a screening tool in primary care because of its extremely low specificity and PPV [8].To bridge the gap between familiar HEp-2 ANA testing and new ANA screening technologies, ACR has recommended that new assays demonstrate the same or improved sensitivity and specificity compared to IFA. In searching the literature and conducting our own studies, we have found that EIA compares well to IFA for ANA screening.
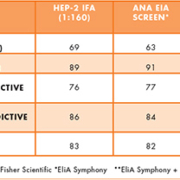
Two studies demonstrate this comparison. The first evaluated 170 pre-selected serum samples from clinically defined patients by comparing the results of IFA testing against an EIA screening assay (EliA® Symphony, Thermo Fisher Scientific) [9]. The results, presented in Table 1, demonstrate comparable performance and efficiency.
The second study examined 388 consecutive samples that were measured using HEp-2 IFA (1:160), an EIA screen and, if that were positive, single ENA differentiation antigens [10]. The study found 84% agreement of results (Table 2), with IFA missing several clinically relevant dsDNA and Ro-negative samples and EIA missing some nucleolar patterns.
Our experience at Baptist Medical Center
We were aware of these studies before introducing the new ELISA-based technology into our laboratory. We also conducted our own testing, to assure our laboratory personnel and ordering physicians that others’ results could be replicated in our setting. Our testing was uniformly encouraging (Table 3). Most notably, the specificity and PPV of EIA testing were greatly improved compared with IFA, and without any appreciable loss in sensitivity.
Still, smooth implementation of any new diagnostic test requires an understanding of its clinical utility and of established practice patterns. Thus, we met with our rheumatologists to explain how the new EIA test compared to the IFA method they were using. Typically they order ANA testing after classifying patients according to established autoimmune disease criteria, as Vos and colleagues did in a study of 328 serum samples (Table 4) [11]. In that study under conditions of daily clinical practice, a similar percentage of patients was found by both methods in each subgroup. Overall, the differences between IFA and EIA were small.
We told the rheumatologists that a positive result on the new ANA-EIA screen indicated the presence of one or more clinically significant antibodies (dsDNA, Sm, U1RNP (RNP 70, A, C), SSA (60 kDa and 52 kDa), SSB, Scl-70, Jo-1, or centromere). And we agreed to offer the HEp-2 IFA methodology on request, should any clinicians need it. We also asked, “If you knew that DNA, centromere and ENA markers were negative, would you still need a pattern and titre?” This led to a discussion of the high number of false-positive results (approximately 25%-30%) [4] found with the traditional IFA-ANA method. Many of these false positives could be associated with the dense fine speckle 70 (DFS70) antigen pattern, which has been found in 33% of ANA-positive healthy individuals but not in ANA-positive sera from patients with SARD [12].
We also informed physicians of the method switch from IFA to EIA screening for our ANA screen, and of the expansion of specific ENA testing for any follow-up testing needs. For positive ANA screen tests, follow-up identification of specific ENA markers can add predictive diagnostic value in their specialist practice [2] A list of frequently asked questions (FAQs) when comparing the two methods was provided to our clinicians as part of this implementation process. These FAQs explained how results would be reported, and alerted physicians to the new testing schedules, including same-day results for tests submitted early in the day. Moreover, we assured them that unlike ANA by IFA, a positive result on an ANA-EIA screen would be positive for an ENA marker, centromere or dsDNA >87% of the time. These steps resulted in a positive reception. We’ve had only a few requests for concurrent IFA testing since we switched over a year ago, and the transition went far better than expected.
Improving laboratory efficiency and clinical utility
Our experience bodes well for the laboratory, physicians and patients. From the laboratory perspective, we can deploy skilled technologists more strategically because of the automation of EIA testing. We currently use less labour to run ANA and dsDNA testing daily than we did for once-a-week testing, and ENA testing has been increased to two or three times per week. We also added CCP and rheumatoid factor testing without requiring any additional labour. Automation has streamlined workflow efficiency, as a technologist can walk away from the Phadia® 250, whereas the manual process required constant attention for a full day. We have also eliminated the complexity and subjectivity of interpreting IFA patterns. Only two technologists were proficient with the complicated manual testing, but more of our personnel possess the skills necessary to operate the new equipment. Automated EIA testing not only provides excellent quality assurance, but also screens for specific, clinically relevant autoantibodies, unlike the broad range of antinuclear antibodies detected by HEp-2 IFA.
From the clinical perspective, providing more specific results in a timely manner allows physicians to focus on the correct diagnosis and treatment without delay because of improved clinical specificity and PPV. False-positive test results from IFA can lead to overdiagnosis and misdiagnosis. Both carry the risk of inappropriate treatment with potent medications, unnecessary referrals, additional laboratory testing, and higher healthcare utilization and costs [13]. Meanwhile, patients may suffer both emotionally and economically. By contrast, we have found that automated and efficient EIA testing for ANA provides reliable, objective information that can be applied clinically with confidence.
References
1. Slater CA, Davis RB, Shmerling RH. Antinuclear antibody testing. A study of clinical utility. Arch Intern Med 1996;156:1421-1425.
2. Peene I, Meheus L, Veys EM, De Keyser F. Detection and identification of antinuclear antibodies (ANA) in a large and consecutive cohort of serum samples referred for ANA testing. Ann Rheum Dis 2001;60:1131-1136.
3. Abeles AM, Abeles M. The clinical utility of a positive antinuclear antibody test result. Am J Med 2013;126(4):342-348.
4. Tan EM, Feltkamp W, Smolen JS, et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum 1997;40(9):1601-1611.
5. Autoimmune diagnostics market to touch $1 bn by 2018. BioSpectrum 20 Sept 2013. http://www.biospectrumasia.com. Accessed Oct. 1, 2013.
6. Agmon-Levin N, Damoiseaux J, Kallenberg C, et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann Rheum Dis [Epub ahed of print Oct. 14, 2013]. doi:101136/annrheumdis-2013-203863.
7. American College of Rheumatology. Antinuclear antibodies (ANA). http://www.rheumatology.org/Practice/Clinical/Patients/Diseases_And_Conditions/Antinuclear_Antibodies_%28ANA%29/. Accessed Nov. 5, 2013.
8. Check W. Making sense of the ANA hodgepodge. CAP Today Sept 2009. http://www.cap.org/apps/portlets/contentViewer/show.do?printFriendly. Accessed Oct. 22, 2013.
9. Gonzalez C, et al. Laboratory screening of connective tissue diseases by a new automated ENA screening assay (EliA Symphony) in clinically defined patients. Clin Chim Acta 2005;359(1-2):109-114.
10. Baronaite R, et al. Comparison of antinuclear antibodies measured with EliA Symphony and immunofluorescence on HEp-2 cells. Poster presented at: the 32nd Scandinavian Congress of Rheumatology, 30 Jan- 3 Feb, 2008, Levi, Finland.
11. Vos PA, Bast EJ, Derksen RH. Cost-effective detection of non-antidouble-stranded DNA antinuclear antibody specificities in daily clinical practice. Rheumatology 2006; 45(5):629-635.
12. Mahler M, Hanly JG, Fritzler MJ. Importance of the dense fine speckled pattern on HEp-2 cells and anti-DFS70 antibodies for the diagnosis of systemic autoimmune diseases. Autoimmunity Rev 2012;11:642-645.
13. Narain S, Richards HB, Satoh M, et al. Diagnostic accuracy for lupus and other systemic autoimmune diseases in the community setting. Arch Intern Med 2004;164:2435-2441.
The author
James D. Peele, PhD
Director of Clinical Chemistry
Baptist Medical Center
Jacksonville, FL
USA