Malaria: rapid and precise diagnosis saves lives
Malaria is an acute and life threatening infection in individuls with no previous immunity. Symptoms are nonspecific and cannot be distinguished from those of influenza or severe bacterial infections. All febrile patients should thus be asked if they have been travelling over the past six months and if so whether the journey was to a malaria endemic area.
Microscopic examination of Giemsa stained thick and thin blood films remains the gold standard, but rapid tests using antigen-capture assays are increasingly used where access to expert microscopy is not available. The appropriate use of rapid tests and their limits are discussed.
by Dr Eskild Petersen
Malaria is caused by a protozoan parasite and five species can infect humans: Plasmodium falciparum, P. vivax, P. ovale, P. malariae and P. knowlesi. Humans are infected by bites of Anopheles mosquitoes and humans are the reservoir hosts except in the case of P. knowlesi, which is transmitted from monkeys and is only seen in South East Asia. Infection with P. falciparum shows the highest mortality and drug resistance is much more common in P. falciparum compared to P. vivax, and not a problem in the other malaria species. P. ovale and P. vivax have persistent liver forms, hypnozoites, which may reactivate, usually within six months after infection, and give rise to a malaria attack.
Malaria in Europe
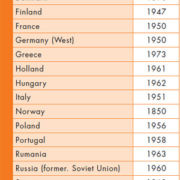
Malaria was endemic in Europe up to the middle of the 20th century [Table 1]. Presently malaria is almost exclusively imported, although a number of Plasmodium vivax cases were seen in Greece in 2011, probably introduced with migrant workers from endemic areas [1]. It is estimated that between 10,000 and 15,000 cases of malaria are imported into Europe every year, which makes the recognition of symptoms and knowledge of appropriate diagnosis important.
A special risk group is immigrants resident in Europe who visit their home countries where malaria is found. The proportion of imported malaria cases in immigrants in Europe has increased from a reported 14% more than 10 years ago to 86% in more recent studies [2]. More than five million African immigrants could be living in Europe, one third of whom are from Sub-Saharan Africa [3], and children of immigrants are particularly at risk [4].
Mortality of imported malaria
The mortality from imported Plasmodium falciparum malaria cases varies from 0.4% in a large cohort from France [5], up to 5% in a recent cluster of cases imported from The Gambia [6]. Malaria infection in non-immunes is an emergency which requires prompt diagnosis and treatment while asymptomatic malaria in immigrants raises other public health issues.
Clinical symptoms
Individuals without immunity, i.e. persons who have not lived in malaria endemic countries for a long time, normally have a febrile illness with an acute onset. The symptoms include fever, malaise, muscle and joint pains, headache and rarely respiratory distress and diarrhoea. Malaria infection can be complicated by bacterial septicaemia. As the infection progresses there can be drowsiness, coma, kidney failure, disseminated intravascular coagulation and low blood pressure, and in the non-immune the mortality of untreated P. falciparum malaria is probably more than 50%.
P. falciparum in non-immunes does not usually follow a regular cyclic pattern and the fact that fever is not cyclic with a 48 or 72 hour cycle cannot be used to exclude malaria. Malaria in non-immunes is a medical emergency and diagnosis should be performed without delay.
In semi-immunes the clinical symptoms may be much more discrete and the development more subtle. Immunity to malaria is not a sterile immunity and a low level parasitaemia is seen in semi-immune individuals, ie. individuals from malaria endemic areas [7]. A special risk group is pregnant women from malaria endemic areas who are at greater risk of clinical malaria during pregnancy [8].
Malaria parasites may persist in asymptomatic immigrants long after their arrival in the host country, and malaria can be transmitted, for instance by blood transfusion or organ transplantation.
Who should be tested for malaria?
Diagnostic tests for malaria infection should be performed in any febrile patients who have a history of exposure, which includes patients with a history of travel in malaria endemic areas, as defined by the WHO.
However, rare modes of transmission mean that patients with fever but without a travel history to endemic areas should be tested. This includes so called ‘airport malaria’ where Anopheles mosquitoes carrying malaria parasites are transported in an airplane, leave the destination and take a blood meal from someone living close to the airport [9,10]. Malaria parasites can be transmitted in blood when sharing instruments used for intravenous drug abuse [11]. Transmission of malaria by blood transfusions from asymptomatic carriers is a huge problem in tropical Africa [12] and febrile patients with a history of receiving blood transfusion from a donor in a malaria endemic area should be suspected of having malaria until it is proven otherwise.
Diagnostic procedures for detecting malaria parasites
Traditionally malaria diagnosis rests on the microscopic examination of thick and thin blood films, but over the past decades, rapid tests based on antigen capture are increasingly used. However, rapid test have pitfalls and parasite density must be measured and followed to monitor the response to treatment. Thus microscopy is still a mandatory skill in institutions taking care of malaria patients.
Microscopic examination of Giemsa stained thick blood films remains the gold standard because it is rapid, easy to perform and sensitive [13] with a sensitivity down to five parasites per microlitre of blood [14]. Microscopy and counting of malaria parasites in patients are mandatory to assess the response to treatment and must be available at centres managing patients with malaria.
Rapid test are available which show a 100% sensitivity down to a parasite density level of 200 parasites per microlitre, equivalent to a parasitaemia of approximately 0.004% [15]. Molecular diagnosis by polymerase chain reaction (PCR) can detect parasites down to a density of 0.01 parasites per microlitre after a lysis procedure, and 1 parasite per microlitre without lysis [16]. However, PCR analysis is not instantly available around the clock so in practice diagnosis relies on rapid diagnostic tests and microscopy of Giemsa stained thick blood films.
Rapid tests are increasingly used in medical centres with limited access to experienced microscopists. However, a rapid test cannot determine the parasite density and rapid tests have limitations. False negative results in patients with very high parasite densities have been described, probably due to the so called ‘pro-zone’ phenomena known from other diagnostic tests [17, 18]. The problem seems to be limited to tests based on detection of the Histidine Rich protein 2, HRP2, and not tests based of detection of Plasmodia LDH, Lactate Dehydrogenase [15, 17]. Mutations in the HRP2 gene may also result is false negative results [19, 20]. All species ie. P. falciparum, vivax, ovale and malariae and P. knowlesi, will be found with tests based on the detection of pan-malarial aldolase antigen aldolase and LDH antigens [21]. P. ovale can be divided in variant and classic P. ovale [22], and variant P. ovale is not picked up in HRP2 based rapid diagnostic tests [23].
Thus clinicians using rapid tests should be instructed that no test so far is 100% reliable. In order to reduce the risk of false negative results, testing should be performed at least twice with 24 hours in between and preferable three times within a 24 hours interval. Variant P. ovale and P. knowlesi infections will be detected by rapid tests, which include those incorporating the pan plasmodia antigens Aldolase or Lactate Dehydrogenase antigens [15, 24]. The latest results of the WHO multicentre evaluation of different rapid diagnostic tests showed that the best performance was found in tests based on a combination of the HRP2 and PLDH proteins [15].
References
1. Danis K et al. Euro Surveill 2011;16:19993.
2. Jelinek T et al. Clin Infect Dis 2002; 34:572-576.
3. Eurostat. European Commission. Katya Vasileva. Population and social conditions. 34/2011. Available at: http://epp.eurostat.ec.europa.eu/cache/ITY_OFFPUB/KS-SF-11-034/EN/KS-SF-11-034-EN.PDF
4. Stäger K et al. Emerg Infect Dis 2009; 15:185–91.
5. Bruneel F et al. PLoS One 2010; 5(10):e13236.
6. Jelinek T et al. Euro Surveill.- 2008;13:19077.
7. Wertheimer ER et al. Emerg Infect Dis 2011;17:1701-3.
8. D’Ortenzio E et al. Emerg Infect Dis 2008;14:323-6.
9. Thang HD et al. Neth J Med 2002;60:441-3.
10. Tatem AJ et al. Malar J 2006;5:57.
11. Chau TT et al. Clin Infect Dis 2002;34:1317-22.
12. Noubouossie D et al. Transfus Med 2012;22:63-7
13. Bowers KM et al. Malar J 2009;8:267.
14. Petersen E et al. Am J Trop Med Hyg 1996; 55:485-489.
15. WHO. Rapid Diagnostic Tests. Results of round 3.http://www.who.int/tdr/publications/tdr-research-publications/rdt_round3/en/index.html Geneva 2011 (Accessed 17th March 2012).
16. Mahajan B et al. Transfusion 2012 Feb 10. doi: 10.1111/j.1537-2995.2011.03541.x. [Epub ahead of print].
17. Luchavez J et al. Malar J 2011;10:286.
18. Gillet P et al. Malar J 2011;10:166.
19. Koita OA et al. Am J Trop Med Hyg 2012;86:194-8.
20. Baker J et al. PLoS One 2011;6:e22593.
21. Chiodini PL et al. Trans R Soc Trop Med Hyg 2007; 101:331-337.
22. Sutherland CJ et al. J Infect Dis 2010;201:1544-1550.
23. Tordrup D et al. Malar J 2011;10:15.
24. Hellemond JJ van et al. Emerg Infect Dis 2009;15:1478–1480.
25. Bruce-Chwatt LJ, Zulueta J de. The rise and fall of malaria in Europe. Oxford University Press. 1980.
The author
Dr Eskild Petersen
Department of Infectious Diseases
Aarhus University Hospital
Skejby
Aarhus
Denmark
Tel +45 7845 2817
e-mail: joepeter@rm.dk