Measurement of serum folate vitamers by LC-MS/MS
The use of liquid chromatography-tandem mass spectrometry (LC-MS/MS) to measure serum folate has been recommended for population monitoring as it allows a more accurate and reproducible measurement than the commonly used protein binding assays. The ability to differentiate between endogenous folate vitamers and folic acid makes LC-MS/MS an extremely valuable tool, not least due to the uncertainty surrounding the potential adverse effects of high concentrations of circulating folic acid.
by Sarah Meadows
Role of folates
Folate is the general term for a water-soluble B vitamin naturally found in foods such as leafy vegetables, legumes, egg yolks, liver and some citrus fruits. Folic acid itself does not occur naturally but it can be found in individuals who take vitamin supplements or eat fortified foods [1, 2]. There are many critical cellular pathways that depend on folate, including DNA, RNA and protein methylation, as well as DNA synthesis and maintenance [2, 3], and because of this there are many health consequences of folate deficiencies among all age groups. These include megaloblastic anemia, depression, cognitive impairment, low birth weight, risk of placental abruption, neural tube defects and other birth defects including orofacial clefts and heart defects [4, 5]. The most widely publicized public health issue surrounding folate is that of low folate during pregnancy causing birth defects associated with the nervous system. Folate is an essential micronutrient during fetal development because of its roles in transmethylation reactions and in synthesis of DNA in growing cells [3]. A significant portion of the 300 000 neural tube defects (NTDs) that occur yearly worldwide are preventable by the periconceptual consumption of folic acid and continue to be a great public health burden globally [2]. The demonstration that periconceptional supplementation with folic acid dramatically reduces the incidence of NTDs has generated considerable clinical and public health interest and has led to fortification with folic acid of the food supply in the United States and some other countries [6]. The recent World Health Organization (WHO) guidelines for ‘Optimal serum and red blood cell folate in women of reproductive age for prevention of neural tube defects’ states that in 2012 an estimated 270 358 deaths globally were attributable to congenital abnormalities during the first 28 days of life. NTDs were one of the most serious and most common abnormalities and increasing awareness of the significance of insufficient folate intake has emphasized the need for identification of accurate biomarkers for large scale assessment of folate status [7].
As well as neural tube closure, B vitamins, including folate, are required for essential brain metabolic pathways and are fundamental in all aspects of brain development and maintenance of brain health throughout the lifecycle. Observational and animal evidence appears to be supportive for a role of maternal folate status in later cognitive performance of the child and there are also studies linking low maternal folate status with a higher incidence of behavioural and emotional problems, inattention and hyperactivity in their offspring [8]. Recent studies have also shown links between low plasma folate and poor cognitive performances in children and adolescents, and similarly a positive association between higher dietary folate intake and academic achievement [8].
Cognitive dysfunction in the elderly (ranging from cognitive impairment to dementia) is also a matter of concern. Brain changes progress long before the diagnosis of dementia is made, and given the increase in life expectancy, the numbers of individuals suffering is set to double by 2025. Therefore it is important to find early biomarkers that would enable timely interventions to delay the onset or slow the progression of the disease. There is emerging evidence suggesting that suboptimal status of folate and metabolically related B vitamins may be linked with cognitive dysfunction and dementia; if this can be slowed or prevented by improving the B vitamin status in healthy older people it could offer a cost effective preventative public health strategy in ageing populations [8].
Evidence showing that supplementation with folic acid protects against NTDs has led to government recommendations, which are in place worldwide, advising all women planning a pregnancy to consume 400 µg/day folic acid from preconception until the end of the first trimester of pregnancy [2, 8–11]. Even with this knowledge, public health campaigns remain largely unsuccessful and limited [2, 9]. Mandatory fortification programmes have been implemented in many countries to improve folate status and reduce high costs associated with prevention programmes such as education campaigns and other interventions that require behavioural change [2]. The Scientific Advisory Committee on Nutrition (SACN) has called for mandatory fortification in the United Kingdom to replace voluntary fortification in a bid to increase the UK population’s folate status [12].
Despite the unequivocal success of folic acid in reducing NTD rates, several studies have questioned whether unmetabolized folic acid in blood may have adverse effects [3, 11]. Concerns have been raised that due to fortification the subsequent increase in folic acid intakes across the population may have harmful effects on health, such as the masking of pernicious anemia, colorectal cancer promotion in people with pre-existing lesions or adverse cognitive effects in the elderly with low vitamin B12 status [4, 9, 11, 13]. Measurement of unmetabolized folic acid has been suggested as a way of monitoring whether folic acid intake is in excess of body requirements [2, 5] and at a time when there are still questions regarding the effects of high levels of folic acid in the blood, the ability to differentiate between this and endogenous folates is valuable.
Serum folate is considered an indicator of recent folate intake whereas red blood cell folate concentrations indicate long term status [7, 10].
Measurement of folates
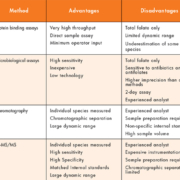
There are several methods currently in use to measure serum folates all with their own advantages and limitations (Table 1). Folate had traditionally been measured using a microbiological assay but, since the 1970s, commercial protein binding assays on automated clinical analysers have been widely used due to both the ease of use of these platforms and the increased throughput they offer. Microbiological assays have not been made obsolete by protein binding assays, as originally expected, due to these assays being well suited to low resource settings [6]. The microbiological assays are considered more accurate as they recover folate vitamers equally and are, therefore, considered the gold standard measurement [14], whereas the protein binding assays generally underestimate folate concentrations due to the different affinities of the folate vitamers for the binding protein used [1, 7].
In contrast to both the microbiological assay and protein binding assays, chromatography techniques are able to differentiate between individual folate species [7] and are now often coupled to mass spectrometers as this method has high sensitivity, specificity and selectivity compared to other detection methods such as fluorimetric or electrochemical detection [6, 7, 15].
The importance of measuring the different folate species is likely to become greater in the future as more information on genetic polymorphisms that affect nutritional status and folate distributions become available [1] and in order to determine the safety of free folic acid in the blood.
The differences seen in results produced by different assays have led the WHO to recommend the standardization of blood vitamin analysis [5, 7]. Initial steps to standardize folate methods began with the development of higher order reference methods that use isotope dilution/ liquid chromatography/tandem mass spectrometry and with recent advances in sample clean up procedures routine methods using LC/MS or LC-MS/MS are becoming more common [5].
Red cell folate is normally calculated using whole blood folate concentrations, serum folate concentrations and hematocrit. However, low concentrations of serum folate within an individual over the course of a month are also indicative of low folate or folate depletion [7, 16]. It has proven to be more technically challenging to measure whole blood folate by LC-MS/MS than it is to measure serum folate, in part because red blood cells first need to be hemolysed to release the polyglutamate folates, which then need to be deconjugated to monoglutamates without any folate loss before being analysed [6, 7]. This has led to whole blood assays only being carried out, commonly by microbiological assays, in specialist laboratories.
LC-MS/MS measurement of folates
The recommendation from an expert and stakeholder workshop for the use of an LC-MS/MS method to measure serum folate for UK population monitoring in 2009 [17] led to the establishment of a assay at the MRC Human Nutrition Unit, Cambridge for the measurement of serum folate in the UK National Diet and Nutrition Survey Rolling Programme (NDNS RP). This was developed from the published method used by the Centers for Disease Control and Prevention, Atlanta, GA for the US National Health and Nutrition Examination Survey (NHANES) [18]. The method described here is for a routine LC-MS/MS method allowing the determination and quantitation of six folate vitamers in serum: tetrahydrofolate (THF); 5-methyltetrahydrofolate (MTHF); 5-formyltetrahydrofolate (FTHF); free folic acid (polyglutamic acid/PGA); 5,10-methenyltetrahydrofolate (5,10 methenylTHF) and an oxidation product of MTHF, MeFox [19].
Samples undergo solid phase extraction, using phenyl columns, to isolate the folate forms in serum samples. Stable isotope-labelled internal standards are added during the extraction step and undergo processing identical to the analytes, thereby normalizing for sample preparation and instrument variability. Analytes are measured using isocratic reversed-phase UPLC prior to electrospray ionization tandem mass spectrometry with a run time of 3.5 minutes. The retention times for all the analytes are very similar and the internal standards are identical to their corresponding analytes, but due to their differing masses, there is clear distinction between them in the assay (Fig. 1). FTHF and MeFox have the same molecular weights and cannot be chromatographically separated, so transitions unique to each form have to be used. The ratio of analyte to internal standard signal is compared to that of a calibration curve to determine analyte concentration.
Recovery is 95.1% for MTHF and >78% for all the other analytes and within batch precision is <7% for all analytes. The calibration graphs are linear, R2 >0.99, for all analytes, from 1 to 100 nmol/L for MTHF and 0.5 to 20 nmol/L for all the other folate forms. Linearity extends above these ranges but these encompass the normal concentrations seen currently in the UK population. Total serum folate concentrations in the UK population lie mainly between 2 and 80 nmol/l. The main folate form in the serum is MTHF, free folic acid and THF are found at concentrations usually <2 nmol/L and MeFox is also found in the majority of samples at concentrations <10 nmol/L. FTHF and CH+THF are rarely found in the serum samples of the UK population.
Tandem mass spectrometry may require operation by experienced personnel but it can provide high throughput measurements in a routine environment. The big disadvantage of this method of analysis to most laboratories is the large financial outlay required to purchase the equipment, but the recent advances in mass spectrometry has led to cheaper and smaller instruments being available and this has led to many more being implemented into routine clinical laboratories.
The use of immunoassays for clinical purposes may still be the preferred option for many routine clinical labs but LC-MS/MS is a more accurate, precise and reliable tool for population studies and research purposes than other available methods.
This work was funded by the Medical Research Council MRC_MC_U105960384.
References
1. Shane B. Folate status assessment history: implications for measurement of biomarkers in NHANES. Am J Clin Nutr. 2011; 94(1): 337S–342S.
2. Crider KS, Bailey LB, Berry RJ. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients, 2011; 3(3): 370–384.
3. Obeid R, Kasoha M, Kirsch SH, Munz W, Herrmann W. Concentrations of unmetabolized folic acid and primary folate forms in pregnant women at delivery and in umbilical cord blood. Am J Clin Nutr. 2010; 92(6): 1416–1422.
4. Smith AD. Folic acid fortification: the good, the bad, and the puzzle of vitamin B-12. Am J Clin Nutr. 2007; 85(1): 3–5.
5. de Benoist B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food Nutr Bull. 2008; 29(2 Suppl): S238–244.
6. Bailey LB. Folate in health and disease, 2nd ed. Taylor & Francis 2010.
7. WHO. In: Guideline: Optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects. WHO 2015. (http://apps.who.int/iris/bitstream/10665/161988/1/9789241549042_eng.pdf)
8. McGarel C, Pentieva K, Strain JJ, McNulty H. Emerging roles for folate and related B-vitamins in brain health across the lifecycle. Proc Nutr Soc. 2015; 74(1): 46–55.
9. Hopkins SM, Gibney MJ, Nugent AP, McNulty H, Molloy AM, Scott JM, Flynn A, Strain JJ, Ward M, Walton J, McNulty BA. Impact of voluntary fortification and supplement use on dietary intakes and biomarker status of folate and vitamin B-12 in Irish adults. Am J Clin Nutr. 2015; 101(6): 1163–1172.
10. Clarke R, Bennett D. Folate and prevention of neural tube defects. BMJ 2014; 349: g4810.
11. European Food Safety Authority (EFSA). Folic acid: an update on scientific developments. EFSA 2010. (https://www.efsa.europa.eu/en/supporting/pub/2e)
12. Scientific Advisory Committee on Nutrition (sacn). Folate and disease prevention. The Stationery Office 2006.
13. Bailey RL, Mills JL, Yetley EA, Gahche JJ, Pfeiffer CM, Dwyer JT, Dodd KW, Sempos CT, Betz JM, Picciano MF. Serum unmetabolized folic acid in a nationally representative sample of adults >/=60 years in the United States, 2001–2002. Food Nutr Res, 2012; 56: DOI: 10.3402/fnr.v56i0.5616.
14. Pfeiffer CM, Hughes JP, Lacher DA, Bailey RL, Berry RJ, Zhang M, Yetley EA, Rader JI, Sempos CT, Johnson CL. Estimation of trends in serum and RBC folate in the U.S. population from pre- to postfortification using assay-adjusted data from the NHANES 1988-2010. J Nutr. 2012; 142(5): 886–893.
15. Wang X, Zhang T, Zhao X, Guan Z, Wang Z, Zhu Z, Xie Q, Wang J, Niu B. Quantification of folate metabolites in serum using ultraperformance liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2014; 962: 9–13.
16. McDowell MA, Lacher DA, Pfeiffer CM, Mulinare J, Picciano MF, Rader JI, Yetley EA, Kennedy-Stephenson J, Johnson CL. Blood folate levels: the latest NHANES results. NCHS Data Brief 2008; 6: 1–8.
17. Duthie SJ, Bird S, Mayer C, Macdonald H. FSA UK: Programme N08: Dietary surveys and nutrients in food: Informed systematic review and critical comparison of analytical methods for the quantification of blood folate status in the population. FSA Website April 2009.
18. Fazili Z, Whitehead RD Jr, Paladugula N, Pfeiffer CM. A high-throughput LC-MS/MS method suitable for population biomonitoring measures five serum folate vitamers and one oxidation product. Anal Bioanal Chem. 2013; 405(13): 4549–60.
19. Meadows S. Multiplex measurement of serum folate vitamers by UPLC-MS/MS. Methods in Molecular Biology 2016 (in press).
The author
Sarah Meadows MSc, CSci
MRC, Elsie Widdowson Laboratory, Cambridge, UK
E-mail: sarah.meadows@mrc-ewl.cam.ac.uk