Measuring cigarette smoke exposure: quantification of cotinine by LC-MS/MS
Smoking is a major cause of morbidity and mortality worldwide. The adverse health effects of chronic cigarette smoke exposure are widely known. Active smoking increases the risk of developing several pathologies including pulmonary disease, cardiovascular disease and cancer. Importantly, the sequelae of smoking also extend to non-smokers via frequent passive inhalation. Accurate measures of cigarette smoke exposure then are required to draw meaningful conclusions about the healthcare risks to both smokers and non-smokers. Cotinine is the major primary metabolite of nicotine and is the biochemical marker of choice for measuring exposure to cigarette smoke.
by Dr A. Dunlop, Dr B. L. Croal and J. Allison
Background
Chronic exposure to tobacco products is amongst the leading causes of preventable morbidity and mortality worldwide, being responsible for approximately 6 million deaths per annum [1]. Typically this involves inhalation of cigarette smoke which contains in excess of 5000 different chemicals; many of these are known toxins and carcinogens [2]. Upon inhalation of cigarette smoke, nicotine is transported to the lungs within tar droplets, dissolving in the alveolar fluid, and is then absorbed into the bloodstream. Following entry into the pulmonary circulation, nicotine quickly travels to the brain – within a matter of seconds – and exerts its pharmacological effects [3].
Nicotine is the addictive component of tobacco products, stimulating dopamine release in the brain and leading to heightened feelings of pleasure and reward [4]. In active smokers this nicotine dependence sustains chronic exposure to the toxins present in cigarette smoke [5]. Active smokers are therefore at increased risk of developing multiple pathologies including pulmonary disease, cardiovascular disease and cancer [6, 7]. Importantly, non-smokers are also at increased risk via involuntary or passive/second-hand smoke (SHS) exposure [8, 9]. Children are particularly susceptible to involuntary exposure, mainly occurring in enclosed spaces such as the parental home/car, via maternal smoking or passive exposure during pregnancy [10]. The adverse health effects of SHS exposure in children include increased risk of miscarriage, sudden infant death syndrome, lower respiratory tract infections, asthma and invasive meningococcal disease [10].
In addition, an emerging area of interest surrounds involuntary exposure via so-called third-hand smoke (THS). THS is a term used to describe the deposits of tobacco smoke that accumulate on surfaces, objects and in dust particles, persisting long after the dispersal of cigarette smoke. There is some evidence to suggest that atmospheric reactions may lead to re-release of smoke-derived toxins into the environment [11]. However, the health risks of THS are not yet known and remain the subject of ongoing research [12].
Assessing cigarette smoke exposure
The healthcare risks associated with cigarette smoking and SHS exposure ensure that smoking status should always be included in any routine clinical assessment. Monitoring of smoking status may also be indicated in specific circumstances, such as epidemiological studies, smoking cessation programmes, lung transplant patients, employee and health/life insurance screening. The most convenient and cost-effective means of assessing cigarette smoke exposure is by self-report. This may occur either during face-to-face consultation with healthcare professionals or often as part of a generic healthcare questionnaire. However self-report is frequently unreliable in estimating smoking status [13].
Moreover, the risk and extent of SHS exposure to non-smokers cannot be adequately assessed using these methods. For example, self-report cannot reliably quantify exposure in those who co-habit and/or socialise with smokers nor can it inform on fetal exposure in maternal smoking. Consequently, cigarette smoke exposure should be accurately quantified by measuring biomarkers to draw meaningful conclusions between smoking status and health outcomes [14, 15].
Biomarkers of cigarette smoke exposure
Numerous biomarkers have been examined in the analysis of cigarette smoke exposure, e.g. carbon monoxide, carboxyhaemoglobin, thiocyanate and polycyclic aromatic hydrocarbons [4]. However, many are non-specific for tobacco use and contribution from other environmental or dietary sources can cause interference [4]. In contrast, nicotine is a more specific marker of cigarette smoke exposure, being derived solely from tobacco [3]. Biochemical measurements of nicotine and its metabolites then are typically used to provide reliable measures of cigarette smoke exposure. Nicotine largely undergoes hepatic metabolism (with a half-life of approximately 2 h) and the plasma of active smokers typically contains 10–50 ng/mL of nicotine [3]. Cotinine is the major breakdown product of nicotine accounting for around 80% of all metabolites [3]. The half-life of cotinine, at around 16 h, is substantially longer than nicotine and plasma levels in active smokers are approximately 250–300 ng/mL [4]. Consequently, cotinine is the preferred biomarker for measuring cigarette smoke exposure.
Quantifying cotinine in biological matrices
A variety of methods have been developed for quantification of cotinine in several biological matrices including urine, blood, saliva and hair [14, 15]. There is good agreement between cotinine levels in plasma/serum and saliva, whilst levels in urine are typically higher [15].
Immunoassay methods have traditionally been used for the detection of cotinine in urine, offering rapid turnaround with minimal sample preparation. In addition, commercially available immunoassay kits are easily integrated into most core automated analysers available in modern clinical laboratories. However, reagent costs are typically high and it would be fair to say that immunoassays may be susceptible to cross-reactivity with other nicotine and cotinine-derived metabolites and thus may be of questionable accuracy [16, 17].
Gas chromatography–mass spectrometry (GC-MS) methods are also available; although sample preparation is typically labour intensive and time consuming, proving impractical for high sample throughput. Not surprisingly, liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods have emerged as the sine qua non for quantification of cotinine in biological fluids.
LC-MS/MS analyses
Liquid chromatography–tandem mass spectrometry (LC-MS/MS) affords the requisite specificity and sensitivity to detect and quantify cotinine at levels encountered throughout the spectrum of cigarette smoke exposure. The majority of recently published methods now routinely quote lower limits of quantification (LLOQ) in the region of <0.5 ng/mL, in both plasma/serum and in urine [15]. Cut-points to distinguish smokers from non-smokers have been variously proposed from 12 ng/mL down to 3 ng/mL, depending on the population [15]. Nevertheless, regular active smokers can be expected to have serum/plasma cotinine levels in marked excess of 100 ng/mL, although non-smokers are usually comfortably below 10 ng/mL.
The majority of LC-MS/MS methods for cotinine have been developed in-house, an important advantage compared with immunoassay techniques. This not only affords flexibility in the choice of matrix to be analysed but also permits the inclusion of more than one analyte in the assay. Thus nicotine, cotinine and various metabolites thereof may be detected in a multiplexed assay. Published guidelines are also widely available to assist in the development and validation of LC-MS/MS methods [18]. The advent of enhanced chromatographic separation techniques, such as ultra-performance liquid chromatography (UPLC), has significantly shortened run times thereby facilitating higher sample throughput. Development of uncomplicated sample preparation procedures has further simplified analyses.
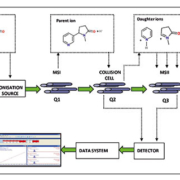
For example, in our own laboratory we recently developed a rapid and straightforward UPLC-MS/MS protocol for the determination of cotinine in plasma (Fig. 1) [19]. Analytical run time was 4 min per sample with a LLOQ of 0.2 ng/mL and the assay was linear from 0.5 to 1000 ng/mL; comfortably covering the concentration range of active and non-smokers (Fig. 2). A simple 5-step automated SPE process was also developed, permitting minimal sample handling and using only water and methanol, both cheap and readily available. To date we have successfully deployed this method for the analyses of two large patient cohorts (each comprising several hundred samples) associated with independent epidemiological studies.
Although the initial outlay for equipment is high, thereafter LC-MS/MS assays can be run relatively cheaply using readily available inexpensive solvents. Furthermore, sample preparation procedures can usually be streamlined/simplified and therefore easily adapted for high-throughput analyses [19]. Matrix effects, chiefly ion suppression, are a particular disadvantage of LC-MS/MS techniques; however careful consideration and troubleshooting during method development can often overcome this issue [20].
Conclusions and future directions
Despite widespread awareness of the adverse effects of tobacco use and increasing public health initiatives to combat this, cigarette smoking continues to be a major global cause of morbidity and mortality and is likely to remain so for the foreseeable future. Accurate quantification of cigarette smoke exposure via biomarkers is therefore an important measure in stratifying the risk of both active and non-smokers.
The need to quantify ever decreasing amounts of nicotine, cotinine and their metabolites in monitoring exposure to tobacco products ensures that LC-MS/MS techniques and modifications thereof remain at the forefront of detection methods in this field. Similarly, as new biomarkers become available which inform on the detrimental health effects of smoking these methods are ideally placed to keep pace, both in research and in clinical laboratories.
The recent emergence of electronic cigarette devices (e-cigarettes) is currently the subject of much debate. E-cigarettes typically deliver nicotine in a vapour generated via heating a liquid that also contains propylene glycol and other additives e.g. flavouring [21]. Exponents propose e-cigarettes as a safer alternative to smoking associated with tobacco combustion and promote the benefits for smoking cessation. However, some healthcare professionals believe that while e-cigarettes are safer, they may still act as a gateway or as a way of prolonging or even enhancing dependency on nicotine. In addition, the long-term health effects of these products are unknown, as is the need to monitor biomarkers such as nicotine and/or cotinine in so-called ‘e-smokers’.
References
1. WHO report on the global tobacco epidemic 2013. http://www.who.int/tobacco/global_report/2013/en/
2. Talhout R, et al. Hazardous compounds in tobacco smoke. Int J Environ Res Public Health 2011; 8(2): 613–628.
3. Hukkanen J, et al. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005; 57(1): 79–115.
4. Benowitz NL, et al. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009; 192(192): 29–60.
5. Berrendero F, et al. Neurobiological mechanisms involved in nicotine dependence and reward: participation of the endogenous opioid system. Neurosci Biobehav Rev. 2010; 35(2): 220–231.
6. Doll R, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ (Clinical research ed.). 2004; 328(7455): 1519.
7. Jha P. Avoidable global cancer deaths and total deaths from smoking. Nature reviews.Cancer. 2011; 9(9): 655–664.
8. Scientific Committee on Tobacco and Health. Secondhand smoke: Review of evidence since 1998. Update of evidence on health effects of secondhand smoke. Department of Health, UK 2004. http://www.smokefreeengland.co.uk/files/scoth_secondhandsmoke.pdf
9. Vardavas CI, Panagiotakos DB. The causal relationship between passive smoking and inflammation on the development of cardiovascular disease: a review of the evidence. Inflamm Allergy Drug Targets 2009; 8(5): 328–333.
10. Action on Smoking and Health. Research Report. Secondhand smoke: the impact on children. March 2014. http://www.ash.org.uk/files/documents/ASH_596.pdf
11. Sleiman M, et al. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. PNAS 2010; 107(15): 6576–6581.
12. Matt GE, et al. Thirdhand tobacco smoke: emerging evidence and arguments for a multidisciplinary research agenda. Environ Health Perspect. 2011; 119(9): 1218–1226.
13. Connor Gorber S, et al. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009; 11(1): 12–24.
14. Florescu A, et al. Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: focus on developmental toxicology. Ther Drug Monitg. 2009; 31(1): 14–30.
15. Avila-Tang E, et al. Assesing secondhand smoke using biological markers. Tob Control 2013; 22: 164–171.
16. Schepers G, Walk RA. Cotinine determination by immunoassays may be influenced by other nicotine metabolites. Arch Toxicol. 1988; 62(5): 395–397.
17. Tate J, Ward G. Interferences in immunoassay. Clin Biochem Rev. 2004; 25(2): 105–120.
18. Honour JW. Development and validation of a quantitative assay based on tandem mass spectrometry. Ann Clin Biochem. 2001; 48(2): 97–111.
19. Dunlop AJ, et al. Determination of cotinine by LC-MS-MS with automated solid-phase extraction. J Chromatogr Sci. 2014; 52(4): 351–356.
20. Matuszewski BK, et al. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003; 75(13): 3019–3030.
21. Grana R, et al. E-cigarettes: a scientific review. Circulation 2014; 129(19): 1972–1986.
The authors
Allan Dunlop1* PhD, Bernard Croal2 MD and James Allison2 BSc
1Department of Clinical Biochemistry Laboratory, Southern General Hospital, Glasgow G51 4TF, UK
2Department of Clinical Biochemistry, Aberdeen Royal Infirmary, Aberdeen AB25 2ZD, UK
*Corresponding author
E-mail: allandunlop@nhs.net