Measuring renal function: traditional methods and iohexol clearance
An accurate measure of glomerular filtration rate (GFR) is required in a variety of clinical situations. Plasma creatinine and creatinine-based equations have served as convenient estimates of GFR but they are subject to limitations. Formal assessment of GFR using clearance methods traditionally necessitates the use of radioactive traces such as 51Cr-EDTA. More recently, iohexol has become increasingly well recognized as an alternative exogenous marker which can now be used routinely, providing a safe, efficient and cost-effective measure.
by Zoe Maunsell and Prof. Tim James
Background
Glomerular filtration rate (GFR) describes the amount of fluid filtered by the glomerulus per unit time, and is therefore a useful indicator of renal function. Expressed in mL/min, it can be accurately measured from the rate of disappearance of an injected substance from the plasma or by collecting urine over a defined time period. The clearance formula, UV/P (where U represents urine concentration, V is urine volume per unit time and P is plasma concentration) can be used to calculate GFR when an ideal marker is used. Such a marker should be freely filtered at the glomerulus, and not secreted, absorbed or metabolized by the renal tubules. Since renal function is proportional to kidney size (which is proportional to body surface area), when estimating GFR, values are usually standardized to an average body surface area of 1.73 m2. Having an accurate yet convenient means to monitor renal function is extremely important in clinical practice. A compromise exists between highly accurate but time consuming, technically difficult reference methods and more accessible, readily available markers of renal function.
Gold standard methods
The ‘gold standard’ for the measurement of GFR is inulin clearance. Inulin is a polysaccharide derived from plants, which can be introduced into the body either by intravenous infusion or bolus dose and its rate of clearance measured. Since inulin is freely filtered by the glomerulus, is not absorbed, secreted or metabolized by the tubules, its elimination is proportional to GFR. Although it is the ‘gold standard’ for assessment of GFR, assay of inulin is technically demanding, and so this technique is not suitable for routine clinical practice.
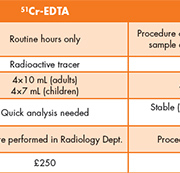
Similarly, the radioactive tracer 51Cr-EDTA can be administered intravenously and its elimination rate monitored. This marker is widely used to measure GFR, but presents a risk to patients and healthcare workers since it involves exposure to ionizing radiation.
Iohexol clearance is an alternative to inulin clearance [1]. Iohexol is an iodine-containing, non-isotopic contrast agent, and studies have shown close agreement between GFR values obtained by iohexol and inulin clearance.
Creatinine and endogenous markers
Creatinine is a widely used clearance marker but suffers from well-described problems including the difficulty and inconvenience of 24-hour urine collection.
GFR has therefore been estimated without urine collection using endogenous markers, the most widely used of which is plasma/serum creatinine. Improvements have been made to the methodology used for creatinine measurement, including standardization of kinetic Jaffe creatinine assays, defining their traceability to isotope-dilution reference assays. However, the Jaffe method remains subject to numerous interferences and therefore laboratories may select the more specific enzymatic creatinine methods. Using creatinine as a marker of GFR has several limitations, including the relationship of creatinine concentration to muscle mass, meaning that estimation of GFR using creatinine alone is particularly a problem in children. It is for this reason that corrections for body surface area have been made. The major limitation of creatinine alone as a marker of GFR is that it is a relatively insensitive marker, and a large decline in renal function can occur before a change in plasma creatinine concentration is observed.
Cystatin C is another endogenous marker that can be used for the estimation of GFR. It is a cysteine protease inhibitor, is produced at a constant rate (independent of muscle mass, sex, age when older than 12 months and inflammatory conditions) and is freely filtered by the kidneys. It is almost completely reabsorbed by the proximal renal tubular cells so that little is normally excreted in the urine. The reciprocal of plasma cystatin C concentration has been shown to be correlated with GFR. Cystatin C has significant advantages over creatinine as a marker of renal function, since increases in serum concentration become apparent with mild renal impairment, such as GFR of 60–90 mL/min, and may be more useful than creatinine in detecting acute kidney injury. Like creatinine, the measurement of cystatin C suffers to some extent from problems with standardization, in particular with reported differences [2] in measured concentrations using turbidimetric versus nephelometric assays by different manufacturers, presumably due to the use of different antibody and detection systems. The availability of an international reference preparation (ERM–DA471/IFCC) is likely to lead to greater agreement between cystatin C methods.
Calculated estimates of GFR
In order to overcome the limitations of measuring creatinine and cystatin C concentrations alone, calculations have been devised. These involve corrections for body surface area, sex, age and ethnicity. Among the most well known are the 4- or 6-parameter MDRD (Modification of Diet in Renal Disease study), Cockroft–Gault (which estimates creatinine clearance) and CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equations for adults and the Schwartz and the Counahan–Barratt prediction equations for children.
The Cockroft–Gault equation [3] provides an estimate of creatinine clearance. Devised in the 1970s, the equation was calculated by examining the mean of two 24-hour creatinine clearances from 249 adult men.
The 4-variable MDRD equation [4] (incorporating creatinine, age, sex and race) is the most widely used in the UK, being reported by laboratories in the form of eGFR, and forms the basis of the staging system for CKD. The formula was developed from the Modification of Diet in Renal Disease study, using data from 1628 patients. The patients had already been diagnosed with CKD, and the study aims were to slow the progression of CKD with a low protein diet and blood pressure control. However, significant limitations to the use of the equation exist, mainly connected with the use of creatinine as a marker of GFR. These include inaccuracies of the equation at extremes of body type such as malnourished or amputee patients and the unsuitability of the equation for use in pregnancy. The formula includes a correction factor for use in African American populations but its validity in other ethnic groups has not been established. The MDRD formula tends to underestimate function at normal GFR, therefore slight reductions in eGFR should not be over-interpreted and reporting eGFR >90mLs/min/1.73m2 is not recommended.
The CKD-EPI equation was developed in 2008 [5] and updated in 2012 [6]. The 2008 equation was developed using data from over 8000 patients from 10 studies. The 2012 versions used over 5000 patients from 13 studies. Importantly, both studies included patients with normal GFR, as well as those with CRF. It was found that the CKD-EPI equations performed better than the MDRD equation, particularly at higher levels of estimated GFR. The limitations of the studies include a limited number of elderly patients and those from ethnic groups other than black race.
In terms of estimation of GFR in children, the Schwartz [7] and Counahan–Barratt [8] equations were developed in the 1970s and have been widely used to estimate GFR from serum creatinine and height (length in infants). The Schwartz equation was modified in 2009 [ 9, 10]. Based on new studies of iohexol clearance, the original formula was found to overestimate GFR. It was postulated that this was in part due to the use of new, standardized creatinine assays. In 2009 the equation was updated, based on studies of 349 patients aged 1–16 years with mild to moderate chronic renal disease. The formula uses creatinine determined using an enzymatic method, urea, height and cystatin C.
Various modifications of these equations, using creatinine, cystatin C [11] or both [12] have been published and optimized in various patient cohorts. When using creatinine-based assays, it is important to know which creatinine assay is being used, since equations have been devised which include different coefficients depending on the methodology used.
Iohexol clearance: a gold standard method in routine use
Oxford University Hospitals NHS Trust has been routinely offering an iohexol clearance service for the measurement of GFR since June 2011. The service is widely used by the hospital’s pediatric service, and is used in a variety of clinical situations, including determining renal function in surgical patients and for chemotherapy dosing. Before the introduction of this service, GFR was assessed using 51Cr EDTA clearance. Although this provided an accurate measurement of GFR, a long waiting list and difficult patient preparation made this technique suboptimal for use in children. In addition, where GFR results are required before administration of chemotherapy agents, timely result availability is critical to prevent delayed treatment. With iohexol clearance we have been able to improve GFR turnaround time compared to 51Cr-EDTA clearance and audit of our first 2 years of service demonstrated that 89% of results were reported within 2 working days and 99% within 3 working days. Iohexol clearance also avoids the use of radioactive isotopes, reducing exposure for patients, carers and hospital staff. Therefore the service has several distinct advantages over conventional isotopic clearance methods (Table 1).
Iohexol clearance is measured according to a standardized protocol, as described previously [13]. In summary, the patient is cannulated, a baseline blood specimen is collected and a standard dose of Omnipaque containing 300 mg iodine/mL is administered through the cannula, (2 mL in patients weighing <40 kg, 5 mL in patients weighing >40 kg). Blood (1 mL) is collected into lithium heparin tubes at 120, 180 and 240 min after iohexol administration.
The iohexol concentration in each specimen is measured by a UPLC (ultra-high performance liquid chromatography) method involving precipitation of plasma samples with equal volumes of perchloric acid and injection of the supernatant onto a Waters 50 x 2.1 mm 1.8 μm reversed phase column with isocratic acetonitrile-based solvent elution. The assay uses a one-point (604 mg/L) calibration through zero. The assay is straighfoward, reliable and rapid; chromatography time is 6 min per specimen. The assay demonstrates excellent inter-assay CVs: 2.2%, 1.9% and 1.9% at 39, 163 and 322 mg/L respectively. The laboratory participates in external quality assessment through the Scandinavian EQUALIS scheme.
The two-point model is used to calculate iohexol clearance, according to the Brochner–Mortensen method [14]. The model assumes a one-compartment model where iohexol is cleared by first-order kinetics. The estimated clearance (Cl ) of the GFR marker is expressed as:
Cl= Q• b/c1 (ml/min)
Where Q = amount of injected marker, b = disappearance rate of marker (min−1), c1= intercept on the y-axis.
The clearance correction for non-immediate mixing of the tracer substance is expressed as:
Cl = 0.990778 x estimated Cl−0.001218• estimated Cl2
Alternatively, single point estimates of GFR at appropriate time points can be calculated using the Jacobsson model. This method can be used in cases where the two-point method cannot be used, for example when contamination has occurred due to poor flushing of lines between sample collection. Here:
GFR = ln(D/VCt) / (t/V + 0.0016)
Where V = volume of distribution in mL (V = 187 x weight in kg + 732), t = time of sampling in min, D = dose of iohexol administered, Ct = concentration of iohexol at time t.
Iohexol clearance is corrected for actual body surface area, using the Dubois and Dubois method, calculated as 0.007184 x (weight in kg)0.425 x (height in cm)0.72, to yield a measure of GFR in mL/min/1.73 m2.
Since initiating the iohexol GFR service in 2011, we have analysed over 400 sets of patient samples. The use of UPLC over HPLC allows for smaller sample volumes to be collected, making the technique particularly suited to pediatrics. Ongoing close liaison with clinicians and nursing staff has revealed that patients and their families have responded favourably to the new procedure and in particular prefer being able to remain on the Daycare Ward throughout the investigation, which is a child-friendly environment.
Iohexol clearance has the added benefit of being significantly less costly than 51Cr-EDTA clearance. The approximate cost is £80 compared to £250 for 51Cr-EDTA.
Conclusions
The measurement of glomerular filtration rate, GFR, is important in a number of clinical scenarios. Much work has been undertaken to develop methods of estimating GFR in order to avoid the time-consuming and relatively invasive formal measurement of renal function using clearance methods. These include the development of eGFR equations based on factors such as plasma creatinine, cystatin C, age, race and body surface area. Equations such as the MDRD and CKD-EPI formulae for adults, and Schwartz and Counahan–Barratt equations for children have been widely used in clinical practice.
However, despite their widespread use, limitations of these equations have been described, including the problems of creatinine-based equations tending to underestimate GFR at normal GFR levels and the issue of creatinine-based formulae being unsuitable for use in patients with non-normal muscle mass.
For the formal measurement of GFR, clearance studies must be performed which have traditionally used radioactive tracers such as 51Chromium-EDTA. However, in recent years the use of iohexol has increased. Thanks to its stability in vitro and relative ease of measurement, the assay of iohexol is rapid, reliable and can be performed on small specimens. This fits in particularly well with the clinical needs of a pediatric service for GFR measurement and is cheaper, safer and more convenient than traditional methods.
References
1. Ng DK, Schwartz GJ, Jacobson LP, Palella FJ, Margolick JB, Warady BA, Furth SL, Muñoz A. Universal GFR determination based on two time points during plasma iohexol disappearance. Kidney Int. 2011; 80: 423–430.
2. Herget-Rosenthal S, Bökenkamp A, Hofmann W. How to estimate GFR-serum creatinine, serum cystatin C or equations? Clin Biochem. 2007; 40: 153–161.
3. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41.
4. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999; 130: 461–470.
5. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, for the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150: 604–612.
6. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, for the CKD-EPI Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367: 20–29.
7. Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 1976; 58: 259–263.
8. Counahan R, Chantler C, Ghazali S, Kirkwood B, Rose F, Baratt TM. Estimation of glomerular filtration rate from plasma creatinine concentration in children. Arch Dis Child 1976; 51: 875–878.
9. Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009; 20: 629–637.
10. Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 2009; 4: 1832–1843.
11. Grubb A, Nyman Un, Björk J, Lindström V, Rippe BG, Christensson A. Simple cystatin C–based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan–Barratt prediction equations for children. Clinical Chemistry 2005; 51: 1420–1431.
12. The Swedish Council on Health Technology Assessment (SBU). Methods to estimate and measure renal function (glomerular filtration rate). Stockholm: The Swedish Council on Health Technology Assessment (SBU). 2012; Yellow 214.
13. James TJ, Lewis AV, Tan GD, Altmann P, Taylor RP, Levy JC. Validity of simplified protocols to estimate glomerular filtration rate using iohexol clearance Ann Clin Biochem. 2007: 44; 369–376.
14. Bröchner-Mortensen J. A simple method for the determination of glomerular filtration rate. Scand J Clin Lab Invest. 1972; 30(3): 271–274.
The authors
Zoe Maunsell* MBiochem MSc DipRCPath and Tim James PhD
Department of Clinical Biochemistry, Oxford University Hospitals NHS Trust, Oxford, UK
*Corresponding author
E-mail: zoe.maunsell@ouh.nhs.uk