Monitoring methotrexate polyglutamate levels in inflammatory bowel disease: where do we stand?
Methotrexate is an established treatment for inflammatory bowel disease, however it is commonly only used as second-line therapy due to concerns over side effects. This article reviews the evidence for using methotrexate polyglutamate levels in the management of rheumatoid arthritis and psoriasis in addition to inflammatory bowel disease with a view to optimizing treatment and helping to prevent toxicity.
by Dr E. L. Johnston, Dr S. C. Fong, Dr A. M. Marinaki, Dr M. Arenas-Hernandez and Dr J. D. Sanderson
Introduction
Methotrexate (MTX) is a folate analogue. It was first used in the 1950s to induce remission in childhood leukemias. Since then its clinical benefit has been widely utilized in the treatment of several inflammatory conditions, including rheumatoid arthritis (RA) and psoriasis, and more recently, inflammatory bowel disease (IBD).
Crohn’s disease (CD) and ulcerative colitis (UC) are chronic inflammatory conditions affecting the gastrointestinal tract, collectively known as IBD. MTX is not as commonly used in the treatment of IBD as other immune modulators, particularly thiopurines. This centres around concerns regarding toxicity and side effects, although in the RA population MTX is frequently used and is considered safe and effective. Monitoring methotrexate, by means of measuring red-cell methotrexate-polyglutamate (MTX-PG) levels, offers the potential to assess adherence along with optimizing dose. However, MTX-PG levels are currently underused because of conflicting evidence regarding interpretation of levels.
Inflammatory bowel disease and methotrexate
The use of methotrexate as a treatment in IBD was initially postulated in the late 1980s when a small study showed an improvement in disease activity indexes, and some histological improvement in the CD cohort, in patients with refractory IBD [1]. Since then, MTX has increasingly been used as a second-line treatment, particularly in those when thiopurine or anti-TNF therapy has failed or not been tolerated.
The European Crohn’s and Colitis Organisation (ECCO) guidelines on the management of CD [2] advise that methotrexate 25 mg/week can be used to treat active CD as an alternative to thiopurines. This is based on a randomized control trial (RCT) in 1995 [3] that showed a significant benefit in taking 25 mg/week of intramuscular (IM) MTX compared with placebo following withdrawal from steroids (39% vs. 19%). It is commonly prescribed orally which is easier for administration and favoured by patients. However, a small study [4] comparing oral to subcutaneous (SC) MTX showed the bioavailability of the oral preparation was variable, despite folic acid use, and favoured SC delivery.
There have been no large studies comparing thiopurines and methotrexate to treat CD and the largest RCT to date looking at the use of MTX as a concomitant immunosuppressant when combined with infliximab, compared to infliximab as monotherapy, showed no benefit in steroid free remission [5].
The evidence to support MTX use in inducing and maintaining remission in patients with UC is less robust with very few good quality RCTs. These studies have shown no benefit over placebo and, therefore, a recent Cochrane review did not support its use [6]. However, two large international RCTs (METEOR and MERIT-UC) looking at the use of MTX for active UC are ongoing.
When MTX is being considered as a treatment option for IBD there are often concerns over the safety of the drug. MTX use requires careful monitoring, particularly of liver function tests because of the risk of hepatotoxicity. However, a retrospective study of its use in CD found it was safe and well tolerated [7]. The commonest side effect was nausea in 22% (17 patients) with only 10% of patients experiencing abnormal liver function tests, resulting in 6% having to stop MTX.
Methotrexate polyglutamate levels
MTX is taken weekly and is commonly administered orally but can be used SC or IM. Despite a stable dose and route of administration there is significant interpatient variability in clinical response and the prevalence of side effects, which is a major drawback of therapy. It has, therefore, long been hypothesized that measuring MTX drug levels could be both a predictor of drug efficacy and a marker of potential toxicity.
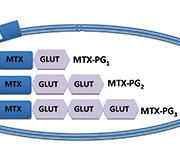
MTX levels peak within hours of oral ingestion and are detectable for less than 24 hours in the serum. Weekly dosing offers no steady-state concentration and, therefore, serum levels are of no clinical benefit. Once in the serum, MTX is transported intracellularly by a reduced folate carrier (RFC) and is changed into a polyglutamated form (MTX-PG1). Further glutamic acid residues (GLUT) are added resulting in up to seven polyglutamates (MTX-PG1–7). This is show in Figure 1.
By using high-performance liquid chromatography it is possible to quantify the seven glutamic residue species in red blood cells [8]. This was first used in children with acute lymphoblastic leukemia [9] and has subsequently been found to correlate with disease activity in other chronic inflammatory conditions. However, MTX-PG6–7 have not previously been detected in RA patients taking MTX [14]; therefore, commonly only MTX-PG1–5 are measured.
Early data suggested that MTX-PG1–2 correlated poorly with drug efficacy in RA; however, the total long-chain polyglutamates (MTX-PG3–5) better reflected the drug effect [8]. MTX-PG3 is the predominant polyglutamate species in red blood cells and is useful to calculate the total long-chain concentrations [10].
Clinical use of methotrexate polyglutamate levels
MTX is widely prescribed for the treatment of RA. Dervieux et al. [10] first looked at the clinical use of MTX-PG measurements in the RA population. In 108 patients who had been on MTX over 3 months, higher MTX-PG levels were associated with a better clinical response to the drug. In particular, patients with a total MTX-PG1–5 that was >60 nmol/L were found to have less tender and swollen joints. The same group expanded their cohort and once again showed that patients with MTX-PG1–5 <60 nmol/L were four times more likely to have a poor response to MTX than those with MTX-PG1–5 >60 nmol/L [11].
Stamp et al. [12] noted large interpatient variability in MTX-PG levels and set out to identify factors that influence levels. Using univariate analysis they found that increased age, impaired renal function, longer duration of treatment and the use of prednisolone resulted in higher MTX-PG levels, whereas smokers generally had lower MTX-PG levels. In contrast to the studies by Dervieux et al., they also surprisingly found that higher doses of MTX were associated with higher MTX-PG levels and increased disease activity [13]. In addition there was no association between MTX-PG levels and adverse effects.
The same group looked at the timing of MTX-PG blood levels and time to steady state [14]. MTX-PG1 was detected 1–2 weeks after first ingestion; however, MTX-PG5 was detected after a median of 7 weeks (range 1–28 weeks). In addition the median time for MTX-PG1–5 to reach steady-state concentration was 27.5 weeks and the median time for MTX-PG1–5 to become undetectable after the last dose was 15 weeks. This highlights that MTX may take up to 6 months to achieve full clinical benefit, which is important to consider when using the levels to assess compliance or to guide dose alteration.
The main trial to be done outside the field of rheumatology was a 55-patient, prospective study into using MTX-PG levels to assess clinical response and compliance in patients with psoriasis [15]. This found the time to steady state of MTX-PG1–5 was between 12–24 weeks, and there was no significant correlation between MTX-PG levels and disease activity.
Methotrexate polyglutamate levels and inflammatory bowel disease
There have been only two studies addressing the potential use of MTX-PG levels in IBD. Egan et al. looked at the total levels when addressing the question of the optimal dose of MTX needed to induce remission in steroid-requiring IBD [16]. They found that subcutaneous initial doses of 15 and 25 mg/week in 32 patients were equally efficacious. In this cohort MTX-PG concentration reached a plateau at around 6–8 weeks after the initiation of therapy and no statistical difference was found between the levels across both doses of the drug. In addition the levels did not correlate with active disease or drug toxicity and did not change significantly after change in MTX dose.
A more recent prospective study from Brooks et al. looked specifically at MTX-PG concentrations in 18 patients with IBD that were on stable doses of MTX [8]. MTX-PG were measured on three occasions and compared to disease activity and reports of toxic side effects. MTX-PG were detected in all the patients and there was little variability in the levels over the study period. Similar to the Stamp et al. RA study [13], higher MTX-PG4&5 were associated with worse disease activity as well as higher toxic effects.
The cohort was small and heterogeneous with different doses of MTX prescribed (median 20 mg/week) and varied administration methods (oral, subcutaneous and via percutaneous endoscopic gastrostomy tube), which is likely to have had a bearing on the results. The data from a similar cohort was presented at Digestive Diseases Week in 2014 [17], which concluded that MTX-PG could be useful in assessing adherence. A non-significant trend showed higher concentrations were associated with active disease, but this may be due to higher doses of MTX being used in those with active disease.
Summary
Methotrexate is an established treatment for IBD. It is an efficacious and well tolerated therapeutic option in CD, particularly when administered SC. More studies are ongoing in the UC population. Measuring MTX-PG levels in RBC has the potential to not only monitor compliance but also correlate with disease activity and toxicity. Two large studies in patients with RA have produced conflicting results but in the small, IBD trials, higher MTX-PG levels, particularly MTX-PG4&5 correlated with increased disease activity and toxicity. It is important, however, to be aware that MTX-PG are influenced by other factors, particularly age and renal function, and may take up to 6 months to reach steady state.
Future trends and developments
Measuring drug levels plays an important role in the management of patients with IBD, as demonstrated by the monitoring of thioguanine nucleotides in those prescribed azathioprine [18]. Measuring MTX-PG offers an exciting step towards individualizing drug treatment and reducing toxicity in those taking MTX. However, at the moment there is a lack of substantial evidence to support the use of measuring MTX-PG levels in IBD, aside from monitoring compliance [19]. A large, prospective trial is warranted to determine clinical benefit before widespread use in the IBD population is advocated.
References
1. Kozarek RA, Patterson DJ, Gelfand MD, et al. methotrexate induces clinical and histological remission in patients with refractory inflammatory bowel disease. Ann Intern Med. 1989; 110: 353–356.
2. Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: Current management. J Crohn’s Colitis 2010; 4: 28–62.
3. Feagan BG, Rochon J, Fedorak RN, et al. Methotrexate in the treatment of Crohn’s disease. New England Journal of Medicine. 1995; 332: 292–297.
4. Kurnik D, Loebstein R, Fishbein E, et al. Bioavailability of oral vs. subcutaneous low-dose methotrexate in patients with Crohn’s disease. Aliment Pharmacol Ther. 2003; 18(1): 57–63.
5. Feagan BG, McDonald JW, Panaccione R, et al. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn’s disease. Gastroenterology 2014; 146(3): 681–688.
6. Chande N, Wang Y, MacDonald JK, et al. Methotrexate for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2014; 8.
7. Chande N, Abdelgadir I, Gregor J. The safety and tolerability of methotrexate for treating patients with Crohn’s disease. J Clin Gastroenterol. 2011; 45: 599–601.
8. Brooks A, Begg E, Zhang M, et al. Red blood cell methotrexate polyglutamate concentrations in inflammatory bowel disease. Ther Drug Monit. 2007; 29: 619–625.
9. Lena N, Imbert AM, Brunet P, et al. Kinetics of methotrexate and its metabolites in red blood cells. Cancer Drug Deliv. 1987; 4(2): 119–127.
10. Dervieux T, Furst D, Lein DO, et al. Polyglutamation of methotrexate with common polymorphisms in reduced folate carrier, aminoimidazole carboxamide ribonucleotide transformylase, and thymidylate synthase are associated with methotrexate effects in rheumatoid arthritis. Arthritis Rheum. 2004; 50(9): 2766–2774.
11. Dervieux T, Furst D, Lein DO, et al. Pharmacogenetic and metabolite measurements are associated with clinical status in patients with rheumatoid arthritis treated with methotrexate: results of a multicentred cross sectional observational study. Ann Rheum Dis. 2005; 64: 1180–1185.
12. Stamp LK, O’Donnell JL, Chapman PT, et al. Determinants of red blood cell methotrexate polyglutamate concentrations in rheumatoid arthritis patients receiving long-term methotrexate treatment. Arthritis Rheum. 2009; 60(8): 2248–2256.
13. Stamp LK, O’Donnell JL, Chapman PT, et al. Methotrexate polyglutamate concentrations are not associated with disease control in rheumatoid arthritis patients receiving long-term methotrexate therapy. Arthritis Rheum. 2010; 62(2): 359–368.
14. Dalrymple JM, Stamp LK, O’Donnell JL, et al. Pharmacokinetics of oral methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 2008; 58(11): 3299–3308.
15. Woolf RT, West SL, Arenas-Hernandez M, et al. Methotrexate polyglutamates as a marker of patient compliance and clinical response in psoriasis: a single-centre prospective study. Br J Dermatol. 2012; 167: 165–173.
16. Egan LJ, Sandborn WJ, Tremaine WJ, et al. A randomised dose-response and pharmacokinetic study of methotrexate for refractory inflammatory Crohn’s disease and ulcerative colitis. Aliment Pharmacol and Ther. 1999; 13: 1597–1604.
17. Ward MG, Fong S, Nasr I, et al. Higher red blood cell methotrexate polyglutamates correlate with increased disease activity, and are useful in assessing adherence. Abstract presented at Digestive Disease Week 2014.
18. Smith M, Blaker, P, Patel C, et al. The impact of introducing thioguanine nucleotide monitoring into an inflammatory bowel disease clinic. Int J Clin Pract. 2013; 67(2): 161–169.
19. Bruns T, Stallmach A. Drug monitoring in inflammatory bowel disease: helpful of dispensable? Dig Dis. 2009; 27: 394–403.
The authors
Emma L. Johnston1 MBBS BSc MRCP, Steven C. Fong1 MBBS MRCP, Anthony M. Marinaki2 PhD, Monica Arenas-Hernandez2 PhD, Jeremy D. Sanderson*1 MD FRCP
1Inflammatory Bowel Disease Centre, Dept of Gastroenterology, Guy’s and St Thomas’ NHS Foundation Trust, London, UK.
2Purine Research Laboratory, Viapath, Guy’s & St. Thomas’ NHS Foundation Trust, London, UK.
*Corresponding author
E-mail: jeremy.sanderson@kcl.ac.uk